NNI-351
NNI-351 is an orally active inhibitor of DYRK1A and neurogenesis enhancer which is under development by NeuroNascent, Inc. for the treatment of Down syndrome, depression, and post-traumatic stress disorder (PTSD).[1][2] As of 2017, it is in the preclinical development stage, and has yet to progress to human clinical trials.[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| Identifiers | |
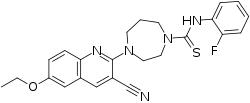
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C24H24FN5OS |
| Molar mass | 449.548 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- http://adisinsight.springer.com/drugs/800029097
- Froestl W, Muhs A, Pfeifer A (2013). "Cognitive enhancers (nootropics). Part 2: drugs interacting with enzymes". J. Alzheimer's Dis. 33 (3): 547–658. doi:10.3233/JAD-2012-121537. PMID 23042218.
External links
- NNI-351 - NeuroNascent, Inc
- NNI-351 - AdisInsight
- Methods and pharmaceutical compositions for treating down syndrome (patent)
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.