Memory T cell
Memory T cells are a subset of infection- and cancer-fighting T cells (also known as a T lymphocyte) that have previously encountered and responded to their cognate antigen; thus, the term antigen-experienced T cell is often applied. Such T cells can recognize foreign invaders, such as bacteria or viruses, as well as cancer cells. Memory T cells have become "experienced" by having encountered antigen during a prior infection, encounter with cancer, or previous vaccination. At a second encounter with the invader, memory T cells can reproduce to mount a faster and stronger immune response than the first time the immune system responded to the pathogen that entered the body. This behaviour is utilized in T lymphocyte proliferation assays, which can reveal exposure to specific antigens.
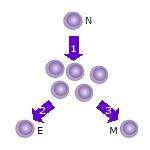
2. Some of the T cell clones will differentiate into effector T cells (E) that will perform the function of that cell (e.g. produce cytokines in the case of helper T cells or invoke cell killing in the case of cytotoxic T cells).
3. Some of the cells will form memory T cells (M) that will survive in an inactive state in the host for a long period of time until they re-encounter the same antigen and reactivate.
Sub-populations
Historically, memory T cells were thought to belong to either the effector or central memory subtypes, each with their own distinguishing set of cell surface markers (see below).[1] Subsequently, numerous additional populations of memory T cells were discovered including tissue-resident memory T (TRM) cells, stem memory TSCM cells, and virtual memory T cells. The single unifying theme for all memory T cell subtypes is that they are long-lived and can quickly expand to large numbers of effector T cells upon re-exposure to their cognate antigen. By this mechanism they provide the immune system with "memory" against previously encountered pathogens. Memory T cells may be either CD4+ or CD8+ and usually express CD45RO and at the same time lack CD45RA.[2]
Memory T cell subtypes:
- Central memory T cells (TCM cells) express CD45RO, C-C chemokine receptor type 7 (CCR7), and L-selectin (CD62L). Central memory T cells also have intermediate to high expression of CD44. This memory subpopulation is commonly found in the lymph nodes and in the peripheral circulation.
- Effector memory T cells (TEM cells) express CD45RO but lack expression of CCR7 and L-selectin. They also have intermediate to high expression of CD44. These memory T cells lack lymph node-homing receptors and are thus found in the peripheral circulation and tissues.[3] TEMRA stands for terminally differentiated effector memory cells re-expressing CD45RA, which is a marker usually found on naive T cells.[4]
- Tissue resident memory T cells (TRM) occupy tissues (skin, lung, gastrointestinal tract, etc.) without recirculating. One cell surface marker that has been associated with TRM is the integrin αeβ7. These cells are thought to play a major role in protective immunity against pathogens.[5][6] Dysfunctional TRM cells have been implicated in autoimmune diseases, such as psoriasis, rheumatoid arthritis, inflammatory bowel disease.[6] Specific to TRM lymphocytes are genes involved in lipid metabolism, being highly active, roughly 20- to 30-fold more active than in other types of T-cells.[6]
- Virtual memory T cells (TVM) differ from the other memory subsets in that they do not originate following a strong clonal expansion event. Thus, although this population as a whole is abundant within the peripheral circulation, individual virtual memory T cell clones reside at relatively low frequencies. One theory is that homeostatic proliferation gives rise to this T cell population. Although CD8 virtual memory T cells were the first to be described,[7] it is now known that CD4 virtual memory cells also exist.[8]
There have been numerous other subpopulations of memory T cells suggested. Investigators have studied Stem memory TSCM cells. Like naive T cells, TSCM cells are CD45RO−, CCR7+, CD45RA+, CD62L+ (L-selectin), CD27+, CD28+ and IL-7Rα+, but they also express large amounts of CD95, IL-2Rβ, CXCR3, and LFA-1, and show numerous functional attributes distinctive of memory cells.[9]
Function
Antigen-specific memory T cells against viruses or other microbial molecules can be found in both TCM and TEM subsets. Although most information is currently based on observations in the cytotoxic T cells (CD8-positive) subset, similar populations appear to exist for both the helper T cells (CD4-positive) and the cytotoxic T cells. Primary function of memory cells is augmented immune response after reactivation of those cells by reintroduction of relevant pathogen into the body. It is important to note that this field is intensively studied and some information may not be available as of yet.
- TCM : TCM lymphocytes have several attributes in common with stem cells, the most important being the ability of self-renewal, mainly because of high level of phosphorylation on key transcription factor, STAT5. In mice, TCM proved to confer more powerful immunity against viruses[10], bacteria[10] and cancer cells[11], compared to TEM lymphocytes in several experimental models.
- TEM : TEM and TEMRA lymphocytes are primarily active as the CD8 variants, thus being mainly responsible for cytotoxic action against pathogens.[12]
- TRM : Because Trm are present over long periods of time in tissues, or more importantly, barrier tissues(epithelium for example), they are crucial for quick response to barrier breach and response to any relevant pathogen present. Among mechanism that are used by TRM to restrict pathogens is the secretion of granzyme B.[13][14]
- TSCM : Those lymphocytes are capable of self-renewal as are the TCM lymphocytes and are also capable of generating both the TCM and TEM subpopulations.[9] Presence of this population in humans is currently under investigation.
- TVM : As of now, the only function apparent in TVM cells is production of various cytokines[15][16], but there are speculations about their influence in subduing unwanted immunological states and their usage in treating autoimmune disorders.[17]
See also
References
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A (October 1999). "Two subsets of memory T lymphocytes with distinct homing potentials and effector functions". Nature. 401 (6754): 708–12. doi:10.1038/44385. PMID 10537110.
- Akbar AN, Terry L, Timms A, Beverley PC, Janossy G (April 1988). "Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells". Journal of Immunology. 140 (7): 2171–8. PMID 2965180.
- Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF (November 2005). "Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets". Journal of Immunology. 175 (9): 5895–903. doi:10.4049/jimmunol.175.9.5895. PMID 16237082.
- Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G (July 2008). "Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people". Immunity & Ageing. 5 (6): 6. doi:10.1186/1742-4933-5-6. PMC 2515281. PMID 18657274.
- Shin H, Iwasaki A (September 2013). "Tissue-resident memory T cells". Immunological Reviews. 255 (1): 165–81. doi:10.1111/imr.12087. PMC 3748618. PMID 23947354.
- "Study highlights possible Achilles' heel in key immune memory cells".
- Lee YJ, Jameson SC, Hogquist KA (February 2011). "Alternative memory in the CD8 T cell lineage". Trends in Immunology. 32 (2): 50–6. doi:10.1016/j.it.2010.12.004. PMC 3039080. PMID 21288770.
- Marusina AI, Ono Y, Merleev AA, Shimoda M, Ogawa H, Wang EA, et al. (February 2017). "+ virtual memory: Antigen-inexperienced T cells reside in the naïve, regulatory, and memory T cell compartments at similar frequencies, implications for autoimmunity". Journal of Autoimmunity. 77: 76–88. doi:10.1016/j.jaut.2016.11.001. PMC 6066671. PMID 27894837.
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. (September 2011). "A human memory T cell subset with stem cell-like properties". Nature Medicine. 17 (10): 1290–7. doi:10.1038/nm.2446. PMC 3192229. PMID 21926977.
- Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. (March 2003). "Lineage relationship and protective immunity of memory CD8 T cell subsets". Nature Immunology. 4 (3): 225–34. doi:10.1038/ni889. PMID 12563257.
- Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, et al. (July 2005). "Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells". Proceedings of the National Academy of Sciences of the United States of America. 102 (27): 9571–6. doi:10.1073/pnas.0503726102. PMC 1172264. PMID 15980149.
- Farber DL, Yudanin NA, Restifo NP (January 2014). "Human memory T cells: generation, compartmentalization and homeostasis". Nature Reviews. Immunology. 14 (1): 24–35. doi:10.1038/nri3567. PMC 4032067. PMID 24336101.
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR (May 2009). "Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus". Nature Immunology. 10 (5): 524–30. doi:10.1038/ni.1718. PMID 19305395.
- Shin H, Iwasaki A (September 2013). "Tissue-resident memory T cells". Immunological Reviews. 255 (1): 165–81. doi:10.1111/imr.12087. PMC 3748618. PMID 23947354.
- White JT, Cross EW, Kedl RM (June 2017). "+ T cells: where they come from and why we need them". Nature Reviews. Immunology. 17 (6): 391–400. doi:10.1038/nri.2017.34. PMC 5569888. PMID 28480897.
- Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC (August 2013). "Virtual memory CD8 T cells display unique functional properties". Proceedings of the National Academy of Sciences of the United States of America. 110 (33): 13498–503. doi:10.1073/pnas.1307572110. PMC 3746847. PMID 23898211.
- Drobek A, Moudra A, Mueller D, Huranova M, Horkova V, Pribikova M, et al. (July 2018). "Strong homeostatic TCR signals induce formation of self-tolerant virtual memory CD8 T cells". The EMBO Journal. 37 (14). doi:10.15252/embj.201798518. PMC 6043851. PMID 29752423.
Further reading
- Janeway, Charles (2005). Immunobiology: the immune system in health and disease. New York: Garland Science. ISBN 978-0-443-07310-6.
- Lichtman, Andrew H.; Abbas, Abul K. (2003). Cellular and molecular immunology. Philadelphia: Saunders. ISBN 978-0-7216-0008-6.