Ipriflavone
Ipriflavone (INN, JAN; brand name Yambolap) is a synthetic isoflavone which may be used to inhibit bone resorption,[2] maintain bone density and to prevent osteoporosis in postmenopausal women.[1] It is not used to treat osteoporosis. It slows down the action of the osteoclasts (bone-eroding cells), possibly allowing the osteoblasts (bone-building cells) to build up bone mass.
 | |
| Clinical data | |
|---|---|
| Trade names | Yambolap |
| Other names | FLI13; 7-Isopropoxyisoflavone[1] |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.125.854 |
| Chemical and physical data | |
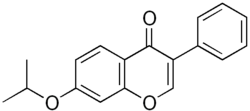
| Formula | C18H16O3 |
| Molar mass | 280.323 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
A clinical trial reported in 2001 that it was not effective in prevention or treatment of osteoporosis.[3]
A double-blind study reveals that ipriflavone might be effective on reducing tinnitus on otosclerosis sufferers.[4]
Ipriflavone has been described as a phytoestrogen.[5] However, this is incorrect, as the drug does not bind to or activate the estrogen receptor and shows no estrogenic effects in postmenopausal women.[6][7] The drug prevents bone loss via mechanisms that are distinct from those of estrogens.[5]
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 651–. ISBN 978-1-4757-2085-3.
- Civitelli, R. (1997). "In Vitro and in Vivo effects of ipriflavone on bone formation and bone biomechanics". Calcified Tissue International. 61: S12–4. doi:10.1007/s002239900378. PMID 9263610.
- Alexandersen, P.; Toussaint, A; Christiansen, C; Devogelaer, JP; Roux, C; Fechtenbaum, J; Gennari, C; Reginster, JY; Ipriflavone Multicenter European Fracture Study (2001). "Ipriflavone in the Treatment of Postmenopausal Osteoporosis: A Randomized Controlled Trial". JAMA. 285 (11): 1482–8. doi:10.1001/jama.285.11.1482. PMID 11255425.
- Sziklai, I; Komora, V; Ribári, O (1992). "Double-blind study on the effectiveness of a bioflavonoid in the control of tinnitus in otosclerosis". Acta Chirurgica Hungarica. 33 (1–2): 101–7. PMID 1343452.
- Arjmandi, B. H.; Birnbaum, R. S.; Juma, S.; Barengolts, E.; Kukreja, S. C. (2000). "The Synthetic Phytoestrogen, Ipriflavone, and Estrogen Prevent Bone Loss by Different Mechanisms". Calcified Tissue International. 66 (1): 61–65. doi:10.1007/s002230050012. ISSN 0171-967X. PMID 10602847.
- Petilli, M.; Fiorelli, G.; Benvenuti, S.; Frediani, U.; Gori, F.; Brandi, M. L. (1995). "Interactions between ipriflavone and the estrogen receptor". Calcified Tissue International. 56 (2): 160–165. doi:10.1007/BF00296349. ISSN 0171-967X. PMID 7736326.
- Melis, Gian Benedetto; Paoletti, A. M.; Cagnacci, A.; Bufalino, L.; Spinetti, A.; Gambacciani, M.; Fioretti, P. (2014). "Lack of any estrogenic effect of ipriflavone in postmenopausal women". Journal of Endocrinological Investigation. 15 (10): 755–761. doi:10.1007/BF03347647. ISSN 0391-4097. PMID 1491124.
External links
- Ipriflavone at PDR Health