Gating (electrophysiology)
In electrophysiology, the term gating refers to the opening (activation) or closing (by deactivation or inactivation) of ion channels.[1] This change in conformation is a response to changes in transmembrane voltage.[2]

When ion channels are in a 'closed' (non-conducting) state, they are impermeable to ions and do not conduct electrical current. When ion channels are in their open state, they conduct electrical current by allowing specific types of ions to pass through them, and thus, across the plasma membrane of the cell. Gating is the process by which an ion channel transitions between its open and closed states.[3]
A variety of cellular changes can trigger gating, depending on the ion channel, including changes in voltage across the cell membrane (voltage-gated ion channels), chemicals interacting with the ion channel (ligand-gated ion channels), changes in temperature,[4] stretching or deformation of the cell membrane, addition of a phosphate group to the ion channel (phosphorylation), and interaction with other molecules in the cell (e.g., G proteins).[5] The rate at which any of these gating processes occurs in response to these triggers are known as the kinetics of gating. Some drugs and many ion channel toxins act as 'gating modifiers' of voltage-gated ion channels by changing the kinetics of gating.[6]
The voltage-gated ion channels of the action potential are often described as having four gating processes: activation, deactivation, inactivation, and reactivation (also called 'recovery from inactivation'). Activation is the process of opening the activation gate, which occurs in response to the voltage inside the cell membrane (the membrane potential) becoming more positive with respect to the outside of the cell (depolarization), and 'deactivation' is the opposite process of the activation gate closing in response to the inside of the membrane becoming more negative (repolarization). 'Inactivation' is the closing of the inactivation gate, and occurs in response to the voltage inside the membrane becoming more positive, but more slowly than activation. 'Reactivation' is the opposite of inactivation, and is the process of reopening the inactivation gate.[7]
These voltage-dependent changes in function are critical for a large number of processes in excitable and nonexcitable cells.[2]
Activation
Voltage-gated ion channels

Voltage-gated ion channels open and close in response to the electrical potential across the cell membrane. Portions of the channel domain act as voltage sensors. As the membrane potential changes, this results in changes in electrostatic forces, moving these voltage-sensing domains. This changes the conformation of other elements of the channel to either the open or closed position.[8] When they move from the closed position to the open position, this is called "activation." Voltage-gated ion channels underlie many of the electrical behaviors of the cell, including action potentials, resting membrane potentials, and synaptic transmission.[9]
Voltage-gated ion channels are often specific to ions, including Na+, K+, Ca2+, and Cl-. Each of these ions plays an important role in the electrical behavior of the cell.[9] The gates also have unique properties with important physiological implications. For example, Na+ channels open and close rapidly, while K+ gates open and close much more slowly. The difference in speed between these channels underlies the depolarization and repolarization phases of the action potential.[10]
Na+ Channels
Voltage Gated Sodium (Na+) channels are significant when it comes to propagating the action potentials in neurons and other excitable cells, mostly being used for the propagation of action potential in axons, muscle fibers and the neural somatodendritic compartment[11]. Sodium(Na+) channels are some of the main ion channels responsible for action potentials.[9] Being complex, they are made of bigger α subunits that are then paired with two smaller β subunits.[11] They contain transmembrane segments known as S1-6. The charged S4 segments are the channels voltage sensors. When exposed to a certain minimum potential difference, the S4 segments move across the membrane.[12] This causes movement of the S4-S5 linker, which causes the S5-S6 linker to twist and opens the channel.[13]
K+ Channels
Potassium (K+) channels play a large role in setting the resting membrane potential.[9] When the cell membrane depolarizes, the intracellular part of the channel becomes positively charged, which causes the channel's open configuration to become a more stable state than the closed configuration. There are a few models of potassium channel activation:
- The sliding helix model posits that the potassium channel opens due to a screwing motion by its S4 helix.
- The paddle model posits that the S3 and S4 helices of the channel form "paddles" that move through the depolarized membrane and pull the S5 helix away from the channel's opening.
- The transport model posits that a focused electric field causes charged particles to move across the channel with only a small movement of the S4 helix.
- The model of coordinated movement of helices posits that the S4 and S5 helices both rotate, and the S4-S5 linker causes the S6 helix to move, opening the channel.
- The consensus model is an average of the above models that helps reconcile them with experimental data.[14]
Ca2+ Channels
Calcium (Ca2+) channels regulate the release of neurotransmitters at synapses, control the shape of action potentials made by sodium channels, and in some neurons, generate action potentials.[9] Calcium channels consist of six transmembrane helices. S4 acts as the voltage sensor by rotating when exposed to certain membrane potentials, thereby opening the channel.[15]
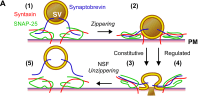
Neurotransmitters are initially stored and synthesized in vesicles at the synapse of a neuron. When an action potential occurs in a cell, the electrical signal reaches the presynaptic terminal and the depolarization causes calcium channels to open, releasing calcium to travel down its electrochemical gradient. This influx of calcium subsequently is what causes the neurotransmitter vesicles to fuse with the presynaptic membrane.[16] The calcium ions initiate the interaction of obligatory cofactor proteins with SNARE proteins to form a SNARE complex.[16] These SNARE complexes mediate vesicle fusion by pulling the membranes together, leaking the neurotransmitters into the synaptic cleft. The neurotransmitter molecules can then signal the next cell via receptors on the post synaptic membrane. These receptors can either act as ion channels or GPCR (G-Protein Coupled Receptors).[17] In general the neurotransmitter can either cause an excitatory or inhibitory response, depending on what occurs at the receptor.
Cl− Channels
Chloride channels are another group of voltage gated ion channels, of which are less understood. They are involved with processes such as skeletal and cardiac smooth muscle, cell volume regulation, the cell cycle, and apoptosis.[18] One major family of chloride proteins are called CLC proteins- common channels and transporters for basic physiological processes in mammals. CLC channels act as slow gated channels; hydrogen ions are exchanged for an influx of chloride ions, allowing the anions to travel via their electrochemical gradient.[19] The voltage dependent C1C-1 chloride channel is homologous dimer which falls under this family, and is seen predominantly in skeletal muscle fibers.[20] With this channel the correct depolarization and repolarization via chloride ions is essential for propagation of an action potential. [18]
Ligand-gated ion channels
Ligand-gated ion channels are found on postsynaptic neurons. By default, they assume their closed conformation. When the presynaptic neuron releases neurotransmitters at the end of an action potential, they bind to ligand-gated ion channels. This causes the channels to assume their open conformation, allowing ions to flow through the channels down their concentration gradient. Ligand-gated ion channels are responsible for fast synaptic transmission in the nervous system and at the neuromuscular junction.[21] Each ligand-gated ion channel has a wide range of receptors with differing biophysical properties as well as patterns of expression in the nervous system.[22]
Inactivation
Inactivation is when the flow of ions is blocked by a mechanism other than the closing of the channel.[23] A channel in its open state may stop allowing ions to flow through, or a channel in its closed state may be preemptively inactivated to prevent the flow of ions.[24] Inactivation typically occurs when the cell membrane depolarize, and ends when the resting potential is restored.[23]
In sodium channels, inactivation appears to be the result of the actions of helices III-VI, with III and IV acting as a sort of hinged lid that block the channel. The exact mechanism is poorly understood, but seems to rely on a particle that has a high affinity for the exposed inside of the open channel.[25] Rapid inactivation allows the channel to halt the flow of sodium very shortly after assuming its open conformation.[26]
Ball and chain inactivation

The ball and chain model, also known as N-type inactivation or hinged lid inactivation, is a gating mechanism for some voltage-gated ion channels. Voltage-gated ion channels are composed of 4 α subunits, one or more of which will have a ball domain located on its cytoplasmic N-terminus.[27] The ball domain is electrostatically attracted to the inner channel domain. When the ion channel is activated, the inner channel domain is exposed, and within milliseconds the chain will fold and the ball will enter the channel, occluding ion permeation.[28] The channel returns to its closed state, blocking the channel domain, and the ball leaves the pore.[29]
Deactivation

Deactivation is the return of an ion channel to its closed conformation. For voltage-gated channels this occurs when the voltage differential that originally caused the channel to open returns to its resting value.[30]
In voltage-gated sodium channels, deactivation is necessary to recover from inactivation.[31]
In voltage gated potassium channels, the reverse is true, and deactivation slows the channel's recovery from activation.[32] The closed conformation is assumed by default, and involves the partial straightening of helix VI by the IV-V linker. The mechanisms that cause opening and closing are not fully understand. The closed conformation appears to be a higher energy conformation than the open conformation, which may also help explain how the ion channel activates.[33]
Quantification
Gating charge can be calculated by solving Poisson's equation. Recent studies have suggested a molecular dynamics simulation-based method to determine gating charge by measuring electrical capacitor properties of membrane-embedded proteins.[2] Activity of ion channels located in the plasma membrane can be measured by simply attaching a glass capillary electrode continuously with the membrane.[34] Other ion channels located in the membranes of mitochondria, lysosomes, and the Golgi apparatus can be measured by an emergent technique that involves the use of an artificial bilayer lipid membrane attached to a 16 electrode device that measures electrical activity.[34]
See also
References
- Alberts, Bruce; Bray, Dennis; Lewis, Julian; Raff, Martin; Roberts, Keith; Watson, James D. (1994). Molecular biology of the cell. New York: Garland. pp. 523–547. ISBN 978-0-8153-1620-6.
- Machtens, Jan-Philipp; Briones, Rodolfo; Alleva, Claudia; de Groot, Bert L.; Fahlke, Christoph (2017-04-11). "Gating Charge Calculations by Computational Electrophysiology Simulations". Biophysical Journal. 112 (7): 1396–1405. Bibcode:2017BpJ...112.1396M. doi:10.1016/j.bpj.2017.02.016. ISSN 0006-3495. PMC 5389965. PMID 28402882.
- Goychuk, Igor; Hänggi, Peter (2002-03-19). "Ion channel gating: A first-passage time analysis of the Kramers type". Proceedings of the National Academy of Sciences of the United States of America. 99 (6): 3552–3556. arXiv:physics/0111187. Bibcode:2002PNAS...99.3552G. doi:10.1073/pnas.052015699. ISSN 0027-8424. PMC 122561. PMID 11891285.
- Cesare P, Moriondo A, Vellani V, McNaughton PA (July 1999). "Ion channels gated by heat". Proc. Natl. Acad. Sci. U.S.A. 96 (14): 7658–63. Bibcode:1999PNAS...96.7658C. doi:10.1073/pnas.96.14.7658. PMC 33597. PMID 10393876.
- Hille, Bertil (2001). Ion channels of excitable membranes. Sunderland, Mass: Sinauer. ISBN 978-0-87893-321-1.
- Waszkielewicz, A.M; Gunia, A; Szkaradek, N; Słoczyńska, K; Krupińska, S; Marona, H (April 2013). "Ion Channels as Drug Targets in Central Nervous System Disorders". Current Medicinal Chemistry. 20 (10): 1241–1285. doi:10.2174/0929867311320100005. ISSN 0929-8673. PMC 3706965. PMID 23409712.
- Ahern, Christopher A.; Payandeh, Jian; Bosmans, Frank; Chanda, Baron (January 2016). "The hitchhiker's guide to the voltage-gated sodium channel galaxy". The Journal of General Physiology. 147 (1): 1–24. doi:10.1085/jgp.201511492. ISSN 0022-1295. PMC 4692491. PMID 26712848.
- Bähring, Robert; Covarrubias, Manuel (2011-02-01). "Mechanisms of closed-state inactivation in voltage-gated ion channels". The Journal of Physiology. 589 (Pt 3): 461–479. doi:10.1113/jphysiol.2010.191965. ISSN 0022-3751. PMC 3055536. PMID 21098008.
- Purves, Dale; Augustine, George J.; Fitzpatrick, David; Katz, Lawrence C.; LaMantia, Anthony-Samuel; McNamara, James O.; Williams, S. Mark (2001). "Voltage-Gated Ion Channels". Neuroscience. 2nd Edition.
- Grider, Michael H.; Glaubensklee, Carolyn S. (2019), "Physiology, Action Potential", StatPearls, StatPearls Publishing, PMID 30844170, retrieved 2019-10-29
- Mantegazza, Massimo; Catterall, William A. (2012), Noebels, Jeffrey L.; Avoli, Massimo; Rogawski, Michael A.; Olsen, Richard W. (eds.), "Voltage-Gated Na+ Channels: Structure, Function, and Pathophysiology", Jasper's Basic Mechanisms of the Epilepsies (4th ed.), National Center for Biotechnology Information (US), PMID 22787615, retrieved 2019-11-03
- Sula, Altin; Booker, Jennifer; Ng, Leo C. T.; Naylor, Claire E.; DeCaen, Paul G.; Wallace, B. A. (2017-02-16). "The complete structure of an activated open sodium channel". Nature Communications. 8 (1): 14205. Bibcode:2017NatCo...814205S. doi:10.1038/ncomms14205. ISSN 2041-1723. PMC 5316852. PMID 28205548.
- Catterall, William A. (2013-11-14). "Structure and function of voltage-gated sodium channels at atomic resolution". Experimental Physiology. 99 (1): 35–51. doi:10.1113/expphysiol.2013.071969. ISSN 0958-0670. PMC 3885250. PMID 24097157.
- Grizel, A. V.; Glukhov, G. S.; Sokolova, O. S. (Oct–Dec 2014). "Mechanisms of Activation of Voltage-Gated Potassium Channels". Acta Naturae. 6 (4): 10–26. PMC 4273088. PMID 25558391.CS1 maint: date format (link)
- Catterall, William A. (August 2011). "Voltage-Gated Calcium Channels". Cold Spring Harbor Perspectives in Biology. 3 (8): a003947. doi:10.1101/cshperspect.a003947. ISSN 1943-0264. PMC 3140680. PMID 21746798.
- Südhof, Thomas C. (January 2012). "Calcium Control of Neurotransmitter Release". Cold Spring Harbor Perspectives in Biology. 4 (1): a011353. doi:10.1101/cshperspect.a011353. ISSN 1943-0264. PMC 3249630. PMID 22068972.
- Yoon, Tae-Young; Lu, Xiaobing; Diao, Jiajie; Lee, Soo-Min; Ha, Taekjip; Shin, Yeon-Kyun (June 2008). "Complexin and Ca 2+ stimulate SNARE-mediated membrane fusion". Nature Structural & Molecular Biology. 15 (7): 707–713. doi:10.1038/nsmb.1446. ISSN 1545-9985. PMC 2493294. PMID 18552825.
- "Chloride channels". British Journal of Pharmacology. 158 (Suppl 1): S130–S134. November 2009. doi:10.1111/j.1476-5381.2009.00503_6.x. ISSN 0007-1188. PMC 2884561.
- Accardi, Alessio; Picollo, Alessandra (August 2010). "CLC channels and transporters: proteins with borderline personalities". Biochimica et Biophysica Acta. 1798 (8): 1457–1464. doi:10.1016/j.bbamem.2010.02.022. ISSN 0006-3002. PMC 2885512. PMID 20188062.
- Imbrici, Paola; Altamura, Concetta; Pessia, Mauro; Mantegazza, Renato; Desaphy, Jean-François; Camerino, Diana Conte (2015-04-27). "ClC-1 chloride channels: state-of-the-art research and future challenges". Frontiers in Cellular Neuroscience. 9: 156. doi:10.3389/fncel.2015.00156. ISSN 1662-5102. PMC 4410605. PMID 25964741.
- Alexander, SPH; Mathie, A; Peters, JA (November 2011). "Ligand-Gated Ion Channels". British Journal of Pharmacology. 164 (Suppl 1): S115–S135. doi:10.1111/j.1476-5381.2011.01649_4.x. ISSN 0007-1188. PMC 3315629.
- "Ligand-Gated Ion Channels". British Journal of Pharmacology. 164 (Suppl 1): S115–S135. 2011-11. doi:10.1111/j.1476-5381.2011.01649_4.x. ISSN 0007-1188. PMC 3315629.
- Bähring, Robert; Covarrubias, Manuel (2011-02-01). "Mechanisms of closed-state inactivation in voltage-gated ion channels". The Journal of Physiology. 589 (Pt 3): 461–479. doi:10.1113/jphysiol.2010.191965. ISSN 0022-3751. PMC 3055536. PMID 21098008.
- Armstrong, Clay M. (2006-11-21). "Na channel inactivation from open and closed states". Proceedings of the National Academy of Sciences. 103 (47): 17991–17996. Bibcode:2006PNAS..10317991A. doi:10.1073/pnas.0607603103. ISSN 0027-8424. PMC 1693860. PMID 17101981.
- Kuo, Chung-Chin; Bean, Bruce P. (1994-04-01). "Na+ channels must deactivate to recover from inactivation". Neuron. 12 (4): 819–829. doi:10.1016/0896-6273(94)90335-2. ISSN 0896-6273. PMID 8161454.
- Yu, Frank H; Catterall, William A (2003). "Overview of the voltage-gated sodium channel family". Genome Biology. 4 (3): 207. doi:10.1186/gb-2003-4-3-207. ISSN 1465-6906. PMC 153452. PMID 12620097.
- "Modulation of K+ channel N-type inactivation by sulfhydration through hydrogen sulfide and polysulfides". rdcu.be. Retrieved 2018-11-22.
- Holmgren, M.; Jurman, M. E.; Yellen, G. (September 1996). "N-type inactivation and the S4-S5 region of the Shaker K+ channel". The Journal of General Physiology. 108 (3): 195–206. doi:10.1085/jgp.108.3.195. ISSN 0022-1295. PMC 2229322. PMID 8882863.
- Bénitah, J. P.; Chen, Z.; Balser, J. R.; Tomaselli, G. F.; Marbán, E. (1999-03-01). "Molecular dynamics of the sodium channel pore vary with gating: interactions between P-segment motions and inactivation". The Journal of Neuroscience. 19 (5): 1577–1585. doi:10.1523/JNEUROSCI.19-05-01577.1999. ISSN 0270-6474. PMC 6782169. PMID 10024345.
- Bähring, Robert; Covarrubias, Manuel (2011-01-28). "Mechanisms of closed-state inactivation in voltage-gated ion channels". The Journal of Physiology. 589 (3): 461–479. doi:10.1113/jphysiol.2010.191965. ISSN 0022-3751. PMC 3055536. PMID 21098008.
- Kuo, Chung-Chin; Bean, Bruce P. (1994-04-01). "Na+ channels must deactivate to recover from inactivation". Neuron. 12 (4): 819–829. doi:10.1016/0896-6273(94)90335-2. ISSN 0896-6273. PMID 8161454.
- Kuo, Chung-Chin (1997-05-15). "Deactivation Retards Recovery from Inactivation in Shaker K+ Channels". The Journal of Neuroscience. 17 (10): 3436–3444. doi:10.1523/JNEUROSCI.17-10-03436.1997. ISSN 0270-6474. PMC 6573675. PMID 9133369.
- Fowler, Philip W.; Sansom, Mark S. P. (2013-05-21). "The pore of voltage-gated potassium ion channels is strained when closed". Nature Communications. 4 (1): 1872. Bibcode:2013NatCo...4.1872F. doi:10.1038/ncomms2858. ISSN 2041-1723. PMC 3674235. PMID 23695666.
- Kamiya, Koki; Osaki, Toshihisa; Nakao, Kenji; Kawano, Ryuji; Fujii, Satoshi; Misawa, Nobuo; Hayakawa, Masatoshi; Takeuchi, Shoji (2018-11-30). "Electrophysiological measurement of ion channels on plasma/organelle membranes using an on-chip lipid bilayer system". Scientific Reports. 8 (1): 17498. Bibcode:2018NatSR...817498K. doi:10.1038/s41598-018-35316-4. ISSN 2045-2322. PMC 6269590. PMID 30504856.