Broad-spectrum antibiotic
A broad-spectrum antibiotic is an antibiotic that acts on the two major bacterial groups, gram-positive and gram-negative,[1] or any antibiotic that acts against a wide range of disease-causing bacteria.[2] These medications are used when a bacterial infection is suspected but the group of bacteria is unknown (also called empiric therapy) or when infection with multiple groups of bacteria is suspected. This is in contrast to a narrow-spectrum antibiotic, which is effective against only a specific group of bacteria.[3] Although powerful, broad-spectrum antibiotics pose specific risks, particularly the disruption of native, normal bacteria and the development of antimicrobial resistance. An example of a commonly used broad-spectrum antibiotic is ampicillin.[3]
Uses
Broad-spectrum antibiotics are properly used in the following situations:[4]
- Empirically, when the causative organism is unknown, but delays in treatment would lead to worsening infection or spread of bacteria to other parts of the body. This occurs, for example, in meningitis, where the patient can become fatally ill within hours if broad-spectrum antibiotics are not initiated.
- For drug-resistant bacteria that do not respond to narrow-spectrum antibiotics.
- In the case of superinfections, where there are multiple types of bacteria causing illness, thus warranting either a broad-spectrum antibiotic or combination antibiotic therapy.
- For prophylaxis in order to prevent bacterial infections occurring. For example, this can occur before surgery, to prevent infection during the operation, or for patients with immunosuppression who are at high-risk for dangerous bacterial infections.
Bacterial targets
Antibiotics are often grouped by their ability to act on different bacterial groups. Although bacteria are biologically classified using taxonomy, disease-causing bacteria have historically been classified by their microscopic appearance and chemical function. The morphology of the organism may be classified as cocci, diplococci, bacilli (also known as "rods"), spiral-shaped or pleomorphic. Additional classification occurs through the organism's ability to take up the Gram stain and counter-stain; bacteria that take up the crystal violet dye stain are referred to as "gram-positive," those that take up the counterstain only are "gram-negative," and those that remain unstained are referred to as "atypical." Further classification includes their requirement for oxygen (ie, aerobic or anaerobic), patterns of hemolysis, or other chemical properties. The most commonly encountered groupings of bacteria include gram-positive cocci, gram-negative bacilli, atypical bacteria, and anaerobic bacteria.[4] Antibiotics are often grouped by their ability to act on different bacterial groups. For example, 1st-generation cephalosporins are primarily effective against gram-positive bacteria, while 4th-generation cephalosporins are generally effective against gram-negative bacteria.
Empiric antibiotic therapy
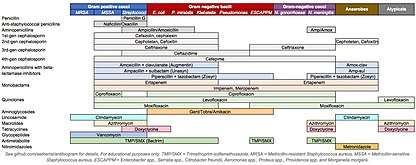
Empiric antibiotic therapy refers to the use of antibiotics to treat a suspected bacterial infection despite lack of a specific bacterial diagnosis. Definitive diagnosis of the species of bacteria often occurs through culture of blood, sputum, or urine, and can be delayed by 24 to 72 hours.[5] Antibiotics are generally given after the culture specimen has been taken from the patient in order to preserve the bacteria in the specimen and ensure accurate diagnosis.[4] Alternatively, some species may be identified through a urine or stool test.[4]
Selection of appropriate treatment
Clinicians often use a step-wise approach to determining appropriate empiric therapy.[5] First, the potential diagnoses are established (for example, lobar pneumonia) and any predisposing risk factors are determined (for example, alcoholism puts patients at risk for Klebsiella pneumonia). Then, the most likely bacterial species for this type of infection are identified (for lobar pneumonia in healthy adults: S. pneumoniae, H. influenzae, etc). Lastly, an antibiotic or group of antibiotics are chosen that are reliably effective against the potential species of bacteria (for example in lobar pneumonia, levofloxacin covers the majority of relevant bacteria). Clinicians often aim to choose empiric antibiotic combinations that cover all appropriate bacteria but minimize coverage of inappropriate bacteria, as to reduce the incidence of antimicrobial resistance (see below). Narrow-spectrum antibiotics have been shown to be just as effective as broad-spectrum alternatives for children with acute bacterial upper respiratory tract infections, and have a lower risk of side effects in children.[6]
A community-wide antibiogram that lists the susceptibility of community-acquired and hospital-acquired bacteria is helpful in guiding empiric therapy.[7] Many professional organizations (for example, the Infectious Disease Society of America) publish guidelines for empiric antibiotic therapy, as do hospitals, with their choices tailored for their specific resistance patterns. Many of these guidelines also offer guidance on antibiotic dose and duration of therapy.
Once a specific species has been identified and its susceptibilities determined, antibiotics can be "narrowed" to a medication which targets a more specific range of bacteria. If no specific species are identified, patients may continue on the empiric regimen.
Risks
Disruption of normal microbiome
There are an estimated 10–100 trillion multiple organisms that colonize the human body.[8] As a side-effect of therapy, antibiotics can change the body's normal microbial content by attacking indiscriminately both the pathological and naturally occurring, beneficial or harmless bacteria found in the intestines, lungs and bladder.[9] The destruction of the body's normal bacterial flora is thought to disrupt immunity, nutrition, and lead to a relative overgrowth in some bacteria or fungi.[10] An overgrowth of drug-resistant microorganisms can lead to a secondary infection such as Clostridium difficile ("C. diff") or candidiasis ("thrush").[3] This side-effect is more likely with the use of broad-spectrum antibiotics, given their greater potential to disrupt a larger variety of normal human flora.[9]
Antimicrobial resistance
After continued exposure to an antibiotic, bacteria may develop changes in their structure or function that make them resistant to the antibiotic. These resistant organisms will live, while the susceptible organisms will die, leaving the population of bacteria entirely resistant to the given antibiotics. For example, after the discovery of penicillin and its subsequent use to treat bacterial infections, bacteria were found to have begun producing an enzyme, penicillinase, which rendered the penicillin molecule inactive. In response to this newly-acquired resistance, newer penicillins were produced that could not be de-activated by penicillinases. This cycle of bacteria evolving resistance to antibiotics and necessitating the development of new antibiotics has been referred to as a "bacterial arms race."[11]
Some bacteria have developed resistance to multiple antibiotics, so-called "superbugs." Methicillin-resistant staphylococcus aureus (MRSA) is one example, and can be life-threatening without the appropriate therapy. Other examples of emerging resistant organisms include vancomycin-resistant enterococcus (VRE), Klebsiella pneumoniae carbapenemase (KPC), and extended-spectrum beta-lactamase producing E coli (ESBL).
In order to combat the rise of antimicrobial resistance, antimicrobial stewardship programs have begun that focus on educating clinicians to improve their use of antibiotics by focusing on evidence-based approaches to minimize resistance.
Examples of broad-spectrum antibiotics
In humans:
- Aminoglycosides (except for streptomycin)
- Ampicillin
- Amoxicillin
- Amoxicillin/clavulanic acid (Augmentin)
- Carbapenems (e.g. imipenem)
- Piperacillin/tazobactam
- Quinolones (e.g. ciprofloxacin)
- Tetracyclines
- Chloramphenicol
- Ticarcillin
- Trimethoprim/sulfamethoxazole (Bactrim)
In veterinary medicine, co-amoxiclav, (in small animals); penicillin & streptomycin and oxytetracycline (in farm animals); penicillin and potentiated sulfonamides (in horses).
References
- Ory, Edwin M. (1963-07-27). "The Use and Abuse of the Broad Spectrum Antibiotics". JAMA: The Journal of the American Medical Association. 185 (4): 273. doi:10.1001/jama.1963.03060040057022. ISSN 0098-7484.
- Clayton L. Thomas, ed. (1993). Taber's Cyclopedic Medical Dictionary (17th ed.). F. A. Davis Co. ISBN 978-0-8036-8313-6.
- S. J. Hopkins (1997). Drugs and Pharmacology for Nurses (12th ed.). Churchill Livingstone. ISBN 978-0-443-05249-1.
- Kasper, Dennis L.; Larry Jameson, J.; Hauser, Stephen L.; Loscalzo, Joseph; Fauci, Anthony S.; Longo, Dan L. (2015-04-08). Harrison's principles of internal medicine. Kasper, Dennis L.,, Fauci, Anthony S., 1940-, Hauser, Stephen L.,, Longo, Dan L. (Dan Louis), 1949-, Jameson, J. Larry,, Loscalzo, Joseph (19th ed.). New York. ISBN 9780071802154. OCLC 893557976.
- Leekha, Surbhi; Terrell, Christine L.; Edson, Randall S. (February 2011). "General Principles of Antimicrobial Therapy". Mayo Clinic Proceedings. 86 (2): 156–167. doi:10.4065/mcp.2010.0639. ISSN 0025-6196. PMC 3031442. PMID 21282489.
- Gerber, Jeffrey S.; Ross, Rachael K.; Bryan, Matthew; Localio, A. Russell; Szymczak, Julia E.; Wasserman, Richard; Barkman, Darlene; Odeniyi, Folasade; Conaboy, Kathryn (December 19, 2017). "Association of Broad- vs Narrow-Spectrum Antibiotics With Treatment Failure, Adverse Events, and Quality of Life in Children With Acute Respiratory Tract Infections". JAMA. 318 (23): 2325–2336. doi:10.1001/jama.2017.18715. ISSN 1538-3598. PMC 5820700. PMID 29260224.
- Halstead, Diane C.; Gomez, Noel; McCarter, Yvette S. (January 2004). "Reality of Developing a Community-Wide Antibiogram". Journal of Clinical Microbiology. 42 (1): 1–6. doi:10.1128/JCM.42.1.1-6.2004. ISSN 0095-1137. PMC 321737. PMID 14715723.
- Ursell, Luke K; Metcalf, Jessica L; Parfrey, Laura Wegener; Knight, Rob (August 2012). "Defining the Human Microbiome". Nutrition Reviews. 70 (Suppl 1): S38–S44. doi:10.1111/j.1753-4887.2012.00493.x. ISSN 0029-6643. PMC 3426293. PMID 22861806.
- E. A. Martin (2003). Oxford Concise Medical Dictionary (6th ed.). Oxford University Press. ISBN 978-0-19-860753-3.
- Rafii, Fatemeh; Sutherland, John B; Cerniglia, Carl E (December 2008). "Effects of treatment with antimicrobial agents on the human colonic microflora". Therapeutics and Clinical Risk Management. 4 (6): 1343–1358. doi:10.2147/tcrm.s4328. ISSN 1176-6336. PMC 2643114. PMID 19337440.
- Reardon, Sara (2015-05-28). "Bacterial arms race revs up". Nature. 521 (7553): 402–403. doi:10.1038/521402a. ISSN 1476-4687. PMID 26017421.