Angomonas deanei
Angomonas deanei is a flagellated trypanosomatid. It is an obligate parasite in the gastrointestinal tract of insects, and is in turn a host to symbiotic bacteria. In fact the bacterial endosymbiont maintains a permanent mutualistic relationship with the protozoan such that it is no longer able to reproduce and survive on its own.[3]
| Angomonas deanei | |
|---|---|
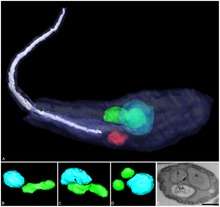 | |
| Three-dimensional reconstruction of Angomonas deanei containing a bacterial endosymbiont (green) near its nucleus (blue).[1] | |
| Scientific classification | |
| Domain: | Eukaryota |
| (unranked): | |
| Phylum: | Euglenozoa |
| Class: | |
| Order: | |
| Genus: | Angomonas |
| Species: | deanei |
| Binomial name | |
| Angomonas deanei (Carvalho 1973) Teixeira & Camargo 2011[2] | |
| Synonyms | |
|
Crithidia deanei Carvalho 1973 | |
The species name was accepted as Crithidia deanei until 2011, when phylogenetic analysis revealed that it belongs to the genus Angomonas, thereby becoming Angomonas deanei. The symbiotic bacterium is a member of the β-proteobacterium that descended from the common ancestor with the genus Bordetella,[2] or more likely, Taylorella.[4] The two organisms have depended on each other so much that the bacterium cannot reproduce and the protozoan can no longer infect insects when they are isolated.[1][5] Thus, this organismal association is a fine model for the evidence of the endosymbiotic theory in nature, which explains the origin of eukaryotic cell organelles such as mitochondria and plastids from individual prokaryotes.[6][7]
Structure
The body of Angomonas deanei is elliptical in shape, with a prominent tail-like flagellum at its posterior end for locomotion. The bacterial endosymbiont is inside its body and is surrounded by two membranes typical of Gram-negative bacteria, but its cell membrane presents unusual features, such as the presence of phosphatidylcholine, a major membrane lipid (atypical of bacterial membranes), and the highly reduced peptidoglycan layer, which shows reduced or absence of rigid cell wall. The cell membrane of the protozoan host contains an 18-domain β-barrel porin, which is a characteristic protein of Gram-negative bacteria.[8] In addition it contains cardiolipin and phosphatidylcholine as the major phospholipids, while sterols are absent.[9] Cardiolipin is a typical lipid of bacterial membranes; phosphatidylcholine, on the other hand, is mostly present in symbiotic prokaryotes of eukaryotic cells. For symbiotic adaptation, the host trypanosome has undergone alterations such as reduced paraflagellar rod, which is required full motility of the bacterial flagella. Yet the paraflagellar rod gene PFR1 is fully functional.[10] The bacteria are known to provide essential nutrients to the host, and provide electron transport system for the production of cellular energy, the ATP molecules through its glycosomes.[1] The bacteria synthesise amino acids,[11] vitamins,[12] and haem[13] for the protozoan. In return the protozoan offers its enzymes for the complete metabolic pathways for the biosysnthesis of amino acids, lipids and nucleotides, that are absent in the bacterium.[14] Phosphatidylinositol, a membrane lipid required for cell-cell interaction, in the bacteria is also synthesised by the protozoan.[15] Thus the two organisms intimately share and exchange their metabolic systems. When the bacterium is killed using antibiotics, the protozoan can no longer infect insects,[5] due to the altered glycosylphosphatidylinositol (gp63) in the protozoan flagellum.[16]
Reproduction
The cellular reproduction shows a strong synergistic adaptation between the bacterium and the protozoan. As each symbiont is each of a single bacterium and a protozoan, and each daughter cell contains the same number, the two cells divide in a coordinated process. The bacterium divides first, followed by the protozoan organelles, and lastly the nucleus.[17]
Evolution
Symbiotic bacteria in the trypanosomatid protozoa are descended from a β-proteobacterium of the genus Bordetella.[18] With A. deanei, the bacteria have co-evolved in a mutualistic relationship characterised by intense metabolic exchanges. The endosymbiont contains enzymes and metabolic precursors that complete essential biosynthetic pathways of the host protozoan, such as those in the urea cycle and the production of haemin and polyamine.[19]
The symbiotic bacterium belongs to β-proteobacterium family Alcaligenaceae. Based on the 16S rRNA gene sequences, it is known that it originated from a common ancestor kinetoplastid with Blastocrithidia species. The two groups are assumed to enter two different host protozoans to evolve into different species. Hence the scientific name (Candidatus) Kinetoplastibacterium crithidii was given to the bacterium.[20] Although it was initially proposed that the bacterium evolved from a common ancestor with members of Bordetella,[2] however, detailed phylogenomic analysis revealed that it is more closely related to members of the genus Taylorella.[4]
References
- Motta MC, Soares MJ, Attias M, Morgado J, Lemos AP, Saad-Nehme J, Meyer-Fernandes JR, De Souza W (1997). "Ultrastructural and biochemical analysis of the relationship of Crithidia deanei with its endosymbiont". Eur J Cell Biol. 72 (4): 370–377. PMID 9127737.
- Teixeira MM, Borghesan TC, Ferreira RC, Santos MA, Takata CS, Campaner M, Nunes VL, Milder RV, de Souza W, Camargo EP (2011). "Phylogenetic validation of the genera Angomonas and Strigomonas of trypanosomatids harboring bacterial endosymbionts with the description of new species of trypanosomatids and of proteobacterial symbionts". Protist. 162 (3): 503–524. doi:10.1016/j.protis.2011.01.001. PMID 21420905.
- Labinfo. "Angomonas deanei". labinfo.lncc.br. National Laboratory of Scientific Computation of the Ministry of Science and Technology, Brazil. Archived from the original on 2013-07-30. Retrieved 2013-07-08.
- Alves JM, Serrano MG, Maia da Silva F, Voegtly LJ, Matveyev AV, Teixeira MM, Camargo EP, Buck GA (2013). "Genome evolution and phylogenomic analysis of Candidatus Kinetoplastibacterium, the betaproteobacterial endosymbionts of Strigomonas and Angomonas". Genome Biol Evol. 5 (2): 338–350. doi:10.1093/gbe/evt012. PMC 3590767. PMID 23345457.
- d'Avila-Levy CM, Silva BA, Hayashi EA, Vermelho AB, Alviano CS, Saraiva EM, Branquinha MH, Santos AL (2005). "Influence of the endosymbiont of Blastocrithidia culicis and Crithidia deanei on the glycoconjugate expression and on Aedes aegypti interaction". FEMS Microbiol Lett. 252 (2): 279–286. doi:10.1016/j.femsle.2005.09.012. PMID 16216441.
- de Souza W, Motta MC (1999). "Endosymbiosis in protozoa of the Trypanosomatidae family". FEMS Microbiol Lett. 173 (1): 1–8. doi:10.1111/j.1574-6968.1999.tb13477.x. PMID 10220875.
- Martin W, Hoffmeister M, Rotte C, Henze K (2001). "An overview of endosymbiotic models for the origins of eukaryotes, their ATP-producing organelles (mitochondria and hydrogenosomes), and their heterotrophic lifestyle". Biol Chem. 382 (11): 1521–1539. doi:10.1515/BC.2001.187. PMID 11767942.
- Andrade Ida S, Vianez-Júnior JL, Goulart CL, Homblé F, Ruysschaert JM, Almeida von Krüger WM, Bisch PM, de Souza W, Mohana-Borges R, Motta MC (2011). "Characterization of a porin channel in the endosymbiont of the trypanosomatid protozoan Crithidia deanei". Microbiology. 157 (Pt 10): 2818–2830. doi:10.1099/mic.0.049247-0. PMID 21757490.
- Palmié-Peixoto IV, Rocha MR, Urbina JA, de Souza W, Einicker-Lamas M, Motta MC (2006). "Effects of sterol biosynthesis inhibitors on endosymbiont-bearing trypanosomatids". FEMS Microbiol Lett. 255 (1): 33–42. doi:10.1111/j.1574-6968.2005.00056.x. PMID 16436059.
- Gadelha C, Wickstead B, de Souza W, Gull K, Cunha-e-Silva N (2005). "Cryptic paraflagellar rod in endosymbiont-containing kinetoplastid protozoa". Eukaryot Cell. 4 (3): 516–525. doi:10.1128/EC.4.3.516-525.2005. PMC 1087800. PMID 15755914.
- Alves, João MP; Klein, Cecilia C; da Silva, Flávia; Costa-Martins, André G; Serrano, Myrna G; Buck, Gregory A; Vasconcelos, Ana Tereza R; Sagot, Marie-France; Teixeira, Marta MG; Motta, Maria Cristina M; Camargo, Erney P (2013). "Endosymbiosis in trypanosomatids: the genomic cooperation between bacterium and host in the synthesis of essential amino acids is heavily influenced by multiple horizontal gene transfers". BMC Evolutionary Biology. 13 (1): 190. doi:10.1186/1471-2148-13-190. PMC 3846528. PMID 24015778.
- Klein, Cecilia C.; Alves, João M. P.; Serrano, Myrna G.; Buck, Gregory A.; Vasconcelos, Ana Tereza R.; Sagot, Marie-France; Teixeira, Marta M. G.; Camargo, Erney P.; Motta, Maria Cristina M.; Parkinson, John (2013). "Biosynthesis of vitamins and cofactors in bacterium-harbouring trypanosomatids depends on the symbiotic association as revealed by genomic analyses". PLoS ONE. 8 (11): e79786. doi:10.1371/journal.pone.0079786. PMC 3833962. PMID 24260300.
- Alves, João M. P.; Voegtly, Logan; Matveyev, Andrey V.; Lara, Ana M.; da Silva, Flávia Maia; Serrano, Myrna G.; Buck, Gregory A.; Teixeira, Marta M. G.; Camargo, Erney P. (2011). "Identification and phylogenetic analysis of heme synthesis genes in trypanosomatids and their bacterial endosymbionts". PLoS ONE. 6 (8): e23518. doi:10.1371/journal.pone.0023518. PMC 3154472. PMID 21853145.
- Motta MC, Martins AC, de Souza SS, Catta-Preta CM, Silva R, Klein CC, de Almeida LG, de Lima Cunha O, Ciapina LP, Brocchi M, Colabardini AC, de Araujo Lima B, Machado CR, de Almeida Soares CM, Probst CM, de Menezes CB, Thompson CE, Bartholomeu DC, Gradia DF, Pavoni DP, Grisard EC, Fantinatti-Garboggini F, Marchini FK, Rodrigues-Luiz GF, Wagner G, Goldman GH, Fietto JL, Elias MC, Goldman MH, Sagot MF, Pereira M, Stoco PH, de Mendonça-Neto RP, Teixeira SM, Maciel TE, de Oliveira Mendes TA, Ürményi TP, de Souza W, Schenkman S, de Vasconcelos AT (2013). "Predicting the proteins of Angomonas deanei, Strigomonas culicis and their respective endosymbionts reveals new aspects of the trypanosomatidae family". PLoS ONE. 8 (4): e60209. doi:10.1371/journal.pone.0060209. PMC 3616161. PMID 23560078.
- de Azevedo-Martins, Allan C; Alves, João MP; Garcia de Mello, Fernando; Vasconcelos, Ana Tereza R; de Souza, Wanderley; Einicker-Lamas, Marcelo; Motta, Maria Cristina M (2015). "Biochemical and phylogenetic analyses of phosphatidylinositol production in Angomonas deanei, an endosymbiont-harboring trypanosomatid". Parasites & Vectors. 8 (1): 247. doi:10.1186/s13071-015-0854-x. PMC 4424895. PMID 25903782.
- d'Avila-Levy CM, Santos LO, Marinho FA, Matteoli FP, Lopes AH, Motta MC, Santos AL, Branquinha MH (2008). "Crithidia deanei: influence of parasite gp63 homologue on the interaction of endosymbiont-harboring and aposymbiotic strains with Aedes aegypti midgut". Exp Parasitol. 118 (3): 345–353. doi:10.1016/j.exppara.2007.09.007. PMID 17945218.
- Motta MC, Catta-Preta CM, Schenkman S, de Azevedo Martins AC, Miranda K, de Souza W, Elias MC (2010). "The bacterium endosymbiont of Crithidia deanei undergoes coordinated division with the host cell nucleus". PLoS ONE. 5 (8): e12415. doi:10.1371/journal.pone.0012415. PMC 2932560. PMID 20865129.
- Du Y, McLaughlin G, Chang KP (1994). "16S ribosomal DNA sequence identities of beta-proteobacterial endosymbionts in three Crithidia species". Journal of Bacteriology. 176 (10): 3081–3084. doi:10.1128/jb.176.10.3081-3084.1994. PMC 205468. PMID 8188611.
- Frossard ML, Seabra SH, DaMatta RA, de Souza W, de Mello FG, Machado Motta MC (2006). "An endosymbiont positively modulates ornithine decarboxylase in host trypanosomatids". Biochem Biophys Res Commun. 343 (2): 443–449. doi:10.1016/j.bbrc.2006.02.168. PMID 16546131.
- Du Y, Maslov DA, Chang KP (1994). "Monophyletic origin of beta-division proteobacterial endosymbionts and their coevolution with insect trypanosomatid protozoa Blastocrithidia culicis and Crithidia spp". Proc Natl Acad Sci U S A. 91 (18): 8437–8441. doi:10.1073/pnas.91.18.8437. PMC 44621. PMID 7521530.