Anabaena
Anabaena is a genus of filamentous cyanobacteria that exist as plankton. They are known for nitrogen-fixing abilities, and they form symbiotic relationships with certain plants, such as the mosquito fern. They are one of four genera of cyanobacteria that produce neurotoxins, which are harmful to local wildlife, as well as farm animals and pets. Production of these neurotoxins is assumed to be an input into its symbiotic relationships, protecting the plant from grazing pressure.
| Anabaena | |
|---|---|
 | |
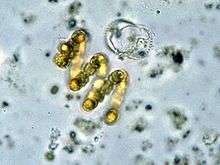
| Anabaena flos-aquae | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Cyanobacteria |
| Class: | Cyanophyceae |
| Order: | Nostocales |
| Family: | Nostocaceae |
| Genus: | Anabaena Bory de Saint-Vincent ex Bornet & Flahault, 1886[1] |
| Species | |
|
A. aequalis | |
A DNA sequencing project was undertaken in 1999, which mapped the complete genome of Anabaena, which is 7.2 million base pairs long. The study focused on heterocysts, which convert nitrogen into ammonia. Certain species of Anabaena have been used on rice paddy fields, proving to be an effective natural fertilizer.
Nitrogen fixation by Anabaena
Under nitrogen-limiting conditions, vegetative cells differentiate into heterocysts at semiregular intervals along the filaments. Heterocyst cells are terminally specialized for nitrogen fixation. The interior of these cells is micro-oxic as a result of increased respiration, inactivation of O2-producing photosystem (PS) II, and formation of a thickened envelope outside of the cell wall. Nitrogenase, sequestered within these cells, transforms dinitrogen into ammonium at the expense of ATP and reductant—both generated by carbohydrate metabolism, a process supplemented, in the light, by the activity of PS I. Carbohydrate, probably in the form of glucose, is synthesized in vegetative cells and moves into heterocysts. In return, nitrogen fixed in heterocysts moves into the vegetative cells, at least in part in the form of amino acids.[2]
The fern Azolla, forms a symbiotic relationship with the cyanobacterium Anabaena azollae, which fixes atmospheric nitrogen, giving the plant access to the essential nutrient. This has led to the plant being dubbed a "super-plant", as it can readily colonise areas of freshwater, and grow at great speed - doubling its biomass in 1.9 days or more,[3]. The typical limiting factor on its growth is phosphorus, abundance of which, due to chemical runoff, often leads to Azolla blooms. Unlike other known plants, the symbiotic microorganism is transferred directly from one generation to the next. This has made Anabaena azollae completely dependent on its host, as several of its genes are either lost or has been transferred to the nucleus in Azolla’s cells.[4]
Primitive vision pigments studied in Anabaena
Anabaena is used as a model organism to study simple vision. The process in which light changes the shape of molecules in the retina, thereby driving the cellular reactions and signals that cause vision in vertebrates, is studied in Anabaena. Anabaena sensory rhodopsin, a specific light-sensitive membrane protein, is central to this research.[5]
References
- Anabaena Bory de Saint-Vincent ex Bornet & Flahault, 1886: 180, 224
- Herrero, Antonia; Flores, Enrique, eds. (2008). The Cyanobacteria: Molecular Biology, Genomics and Evolution (1st ed.). Caister Academic Press. ISBN 978-1-904455-15-8.
- Iwao Watanabe, Nilda S.Berja (1983). "The growth of four species of Azolla as affected by temperature". Aquatic Botany. 15 (2): 175–185. doi:10.1016/0304-3770(83)90027-X.
- The Arctic Azolla event - The Geological Society
- Schapiro, Igor (May 2014). "Ultrafast photochemistry of Anabaena Sensory Rhodopsin: Experiment and theory". Biochimica et Biophysica Acta. 1837 (5): 589–597. doi:10.1016/j.bbabio.2013.09.014. PMID 24099700. Retrieved 2014-06-25.
Further reading
- Mishra, Yogesh; Bhargava, Poonam; Chaurasia, Neha; Rai, Lal Chand (2009). "Proteomic evaluation of the non-survival of Anabaena doliolum (Cyanophyta) at elevated temperatures". European Journal of Phycology. 44 (4): 551–65. doi:10.1080/09670260902947001.
- Eduardo Romero-Vivas, Fernando Daniel Von Borstel, Claudia Perez-Estrada, Darla Torres-Ariño, Francisco Juan Villa-Medina, Joaquin Gutierrez (2015) On-water remote monitoring robotic system for estimating patch coverage of Anabaena sp. filaments in shallow water ; Environ. Sci.: Processes Impacts 04/2015; DOI:10.1039/C5EM00097A
External links
Guiry, M.D.; Guiry, G.M. (2008). "Anabaena". AlgaeBase. World-wide electronic publication, National University of Ireland, Galway.