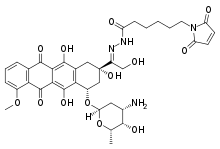
Aldoxorubicin
Aldoxorubicin (INNO-206) is a tumor-targeted doxorubicin conjugate in development by CytRx. Specifically, it is the (6-maleimidocaproyl) hydrazone of doxorubicin. Essentially, this chemical name describes doxorubicin attached to an acid sensitive linker (N-ε-maleimidocaproic acid hydrazide, or EMCH).
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ECHA InfoCard | 100.244.879 |
| Chemical and physical data | |
| Formula | C37H42N4O13 |
| Molar mass | 750.758 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The proposed mechanism of action is as follows:
- After administration, aldoxorubicin rapidly binds endogenous circulating albumin through the EMCH linker.
- Circulating albumin preferentially accumulates in tumors, bypassing uptake by other non-specific sites including heart, bone marrow and gastrointestinal tract.
- Once albumin-bound aldoxorubicin reaches the tumor, the acidic environment of the tumor causes cleavage of the acid sensitive linker.
- Free doxorubicin is released at the site of the tumor.
Clinical trials
Five phase I trials for safety characterization have been completed. Several phase II and III trials are underway.
Phase II
As of January 2017, there are 6 phase II clinical trials in progress:
- Second-line therapy for patients with glioblastoma[1]
- Treatment of HIV-positive patients with Kaposi's sarcoma[2]
- Combination therapy of ifosfamide and aldoxorubicin for treatment of metastatic or locally advanced sarcoma[3]
- Comparison of aldoxorubicin to the gold-standard treatment, topotecan, for metastatic small cell lung cancer[4]
- Treatment of advanced or metastatic pancreatic ductal adenocarcinoma[5]
- Comparison of aldoxorubicin and doxorubicin for patients with metastatic or locally advanced carcinoma[6]
References
- https://clinicaltrials.gov/ct2/show/NCT02014844
- https://clinicaltrials.gov/ct2/show/NCT02029430
- https://clinicaltrials.gov/ct2/show/NCT02235701
- https://clinicaltrials.gov/ct2/show/NCT02200757
- https://clinicaltrials.gov/ct2/show/NCT01580397
- https://clinicaltrials.gov/ct2/show/NCT01514188
- https://clinicaltrials.gov/ct2/show/NCT02049905?
- http://www.streetinsider.com/Conference+Calls/CytRx+(CYTR)+Announces+Statistically+Significant+Data+from+Aldoxorubicin+Phase+3+in+rr+STS/12289769.html
- Kratz, F; Azab, S; Zeisig, R; Fichtner, I; Warnecke, A (Jan 2013). "Evaluation of combination therapy schedules of doxorubicin and an acid-sensitive albumin-binding prodrug of doxorubicin in the MIA PaCa-2 pancreatic xenograft model". Int J Pharm. 441 (1–2): 499–506. doi:10.1016/j.ijpharm.2012.11.003. PMID 23149257.
- Walker, L; Perkins, E; Kratz, F; Raucher, D (Oct 2012). "Cell penetrating peptides fused to a thermally targeted biopolymer drug carrier improve the delivery and antitumor efficacy of an acid-sensitive doxorubicin derivative". Int J Pharm. 436 (1–2): 825–32. doi:10.1016/j.ijpharm.2012.07.043. PMC 3465682. PMID 22850291.
- Kratz, F; Warnecke, A (Dec 2012). "Finding the optimal balance: challenges of improving conventional cancer chemotherapy using suitable combinations with nano-sized drug delivery systems". J Control Release. 164 (2): 221–35. doi:10.1016/j.jconrel.2012.05.045. PMID 22705248.
- Sanchez, E; Li, M; Wang, C; Nichols, CM; Li, J; Chen, H; Berenson, JR (Jul 2012). "Anti-myeloma effects of the novel anthracycline derivative INNO-206". Clin. Cancer Res. 18 (14): 3856–67. doi:10.1158/1078-0432.CCR-11-3130. PMID 22619306.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.