We need you! Join our contributor community and become a WikEM editor through our open and transparent promotion process.
Hydrofluoric acid
From WikEM
Contents
Background
- Used in both commercial and home setting
- Rust remover (most common home use)
- Glass etching, chrome and other metal cleaning, petroleum processing
- Oral ingestion has very high mortality rate
Clinical Features
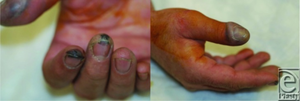
- Skin
- Ophthalmic
- Eye pain
- Erythema
- Ingestion
- Inhalation
- Shortness of breath
- Throat pain/burning
- Signs/symptoms of hypocalcemia and hypomagnesemia
- Can lead to QTc interval prolongation and cardiac arrhythmias, the primary cause of death in HF burns
- Onset and severity of symptoms correlated with concentration
- Dilute solutions (<20%) may have delayed onset up to 24hr post-exposure
- Moderate solutions (20-50%) develop symptoms within 1-8hr
- Concentrated solutions (>50%) develop symptoms immediately
- These patients are at highest risk for systemic toxicity/death
- Pain immediately (even if wound appears minor) implies severe injury
- Burn itself is usually relatively minor
- Toxicity caused by binding of calcium
Differential Diagnosis
Caustic Burns
- Caustic ingestion
- Caustic eye exposure (Caustic keratoconjunctivitis)
- Caustic dermal burn
- Airbag-related burns
- Hydrofluoric acid
- Tar burn
- Cement burn
Evaluation
- Trend calcium and potassium levels
- HF acid chelates calcium and poisons the Na+/K+ pump
- Order serial chemistries, ECGs
- Expect hypocalcemia and hyperkalemia
- Obtain other electrolytes including magnesium
- Can get hypomagnesemia
Management
- Remove soiled clothing and irrigate thoroughly
Cutaneous Burns
Minor injuries (<50 cm2 from dilute solutions <20%)
- Application of gel paste of Ca gluconate or benzalkonium Cl
- Rub into affected area for 10-15min with pain relief being used as end-point of treatment
- Calcium gel is commercially available (found in industrial first-aid kits)
- Calcium gel can be made:
- Mix calcium gluconate powder 3.5gm with 150mL water-soluble lubricant OR
- Mix 25mL 10% calcium gluconate solution with 75mL water-soluble lubricant
- Benzalkonium Cl is commercially available
- If calcium gluconate is not available calcium chloride can be used
Severe injuries
- Treat with intradermal injections of 5% calcium gluconate
- Prepare by diluting conventional 10% Ca gluconate with sterile NS in 1:1 ratio
- Inject in and around the burned area in amount not to exceed 0.5mL per cm2
Refractory injuries
- Treat with intra-arterial infusion of calcium gluconate
- Deliver via arterial line placed proximal to injury in the same limb
- Infuse 10mL of 10% Ca gluconate dilued in 40mL of NS or D5water over 4 hr
Ocular burns
- Irrigate with saline for at least 5 min
- Anesthetic as required
- If persistent pain administer 1% calcium gluconate to eye
- Consult ophthalmology due to irritation effect of calcium salts to eye
- Dilute 10% calcium gluconate with normal saline
Ingestion
- If <1hr of ingestion place NG tube, suction, gastric lavage
- Follow lavage by 300mL 10% Ca gluconate down NGT
- Provide aggressive IV supplementation if ECG signs of hypoCa or hyperK
- Consider intubation
Inhalation
- 100% O2 by facemask
- nebulized 2.5% calcium gluconate
- Follow ECG, electrolytes, and vitals
- Low threshold for obs/admission
Systemic toxicity
- May see QTc prolongation, cardiac arrhythmia, or obvious systemic illness
- Administer calcium gluconate 100mg IV (10 mL of a 10 percent solution) over 2-3 minutes
- May also need to replete magnesium (4g IV over 20 minutes)
- Treat hyperkalemia as needed
Disposition
- Consultation with poison center and burn center transfer per Burn center criteria
- Admission for all patients with arrhythmia on ECG or severe electrolyte disturbance
See Also
References
- Levine MD, Zane R: Chemical Injuries, in Marx JA, Hockberger RS, Walls RM, et al (eds): Rosen’s Emergency Medicine: Concepts and Clinical Practice, ed 8. St. Louis, Mosby, Inc., 2013, (Ch) 64: pp 818-822.