Visual cortex
The visual cortex of the brain is that part of the cerebral cortex which processes visual information. It is located in the occipital lobe. Visual nerves run straight from the eye to the primary visual cortex to the visual association cortex.
| Visual cortex | |
|---|---|
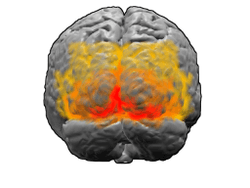 View of the brain from behind. Red = Brodmann area 17 (primary visual cortex); orange = area 18; yellow = area 19 | |
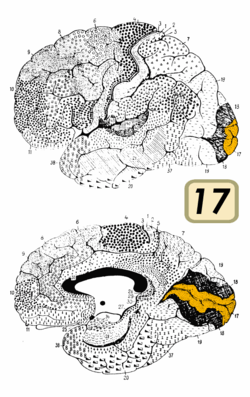 Brain shown from the side, facing left. Above: view from outside, below: cut through the middle. Orange = Brodmann area 17 (primary visual cortex) | |
| Details | |
| Identifiers | |
| Latin | Cortex visualis |
| MeSH | D014793 |
| NeuroLex ID | nlx_143552 |
| FMA | 242644 |
| Anatomical terms of neuroanatomy | |
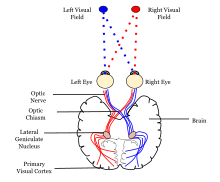
Visual information coming from the eye goes through the lateral geniculate nucleus in the thalamus and then reaches the visual cortex. The part of the visual cortex that receives the sensory inputs from the thalamus is the primary visual cortex, also known as visual area 1 (V1, Brodmann area 17), and the striate cortex. The extrastriate areas consist of visual areas 2 (V2, Brodmann area 18), 3, 4, and 5 (V3, V4, V5, all Brodmann area 19).[1]
Both hemispheres of the brain contain a visual cortex; the visual cortex in the left hemisphere receives signals from the right visual field, and the visual cortex in the right hemisphere receives signals from the left visual field.
Introduction
The primary visual cortex (V1) is located in and around the calcarine fissure in the occipital lobe. Each hemisphere's V1 receives information directly from its ipsilateral lateral geniculate nucleus that receives signals from the contralateral visual hemifield.
Neurons in the visual cortex fire action potentials when visual stimuli appear within their receptive field. By definition, the receptive field is the region within the entire visual field that elicits an action potential. But, for any given neuron, it may respond best to a subset of stimuli within its receptive field. This property is called neuronal tuning. In the earlier visual areas, neurons have simpler tuning. For example, a neuron in V1 may fire to any vertical stimulus in its receptive field. In the higher visual areas, neurons have complex tuning. For example, in the inferior temporal cortex (IT), a neuron may fire only when a certain face appears in its receptive field.
The visual cortex receives its blood supply primarily from the calcarine branch of the posterior cerebral artery.
Psychological model of the neural processing of visual information
Ventral-dorsal model
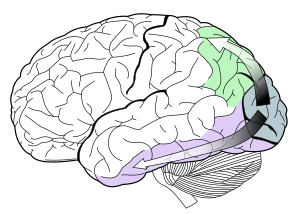
V1 transmits information to two primary pathways, called the ventral stream and the dorsal stream.[2]
- The ventral stream begins with V1, goes through visual area V2, then through visual area V4, and to the inferior temporal cortex (IT cortex). The ventral stream, sometimes called the "What Pathway", is associated with form recognition and object representation. It is also associated with storage of long-term memory.
- The dorsal stream begins with V1, goes through Visual area V2, then to the dorsomedial area (DM/V6) and medial temporal area (MT/V5) and to the posterior parietal cortex. The dorsal stream, sometimes called the "Where Pathway" or "How Pathway", is associated with motion, representation of object locations, and control of the eyes and arms, especially when visual information is used to guide saccades or reaching.
The what vs. where account of the ventral/dorsal pathways was first described by Ungerleider and Mishkin.[3]
More recently, Goodale and Milner extended these ideas and suggested that the ventral stream is critical for visual perception whereas the dorsal stream mediates the visual control of skilled actions.[4] It has been shown that visual illusions such as the Ebbinghaus illusion distort judgements of a perceptual nature, but when the subject responds with an action, such as grasping, no distortion occurs.[5]
Work such as the one from Scharnowski and Gegenfurtner[6] suggests that both the action and perception systems are equally fooled by such illusions. Other studies, however, provide strong support for the idea that skilled actions such as grasping are not affected by pictorial illusions[7][8] and suggest that the action/perception dissociation is a useful way to characterize the functional division of labor between the dorsal and ventral visual pathways in the cerebral cortex.[9]
Primary visual cortex (V1)
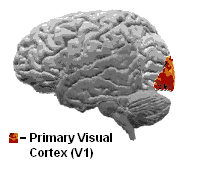
The primary visual cortex is the most studied visual area in the brain. In mammals, it is located in the posterior pole of the occipital lobe and is the simplest, earliest cortical visual area. It is highly specialized for processing information about static and moving objects and is excellent in pattern recognition.
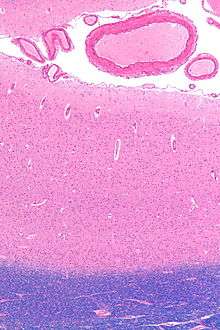
The functionally defined primary visual cortex is approximately equivalent to the anatomically defined striate cortex. The name "striate cortex" is derived from the line of Gennari, a distinctive stripe visible to the naked eye[10] that represents myelinated axons from the lateral geniculate body terminating in layer 4 of the gray matter.
The primary visual cortex is divided into six functionally distinct layers, labeled 1 to 6. Layer 4, which receives most visual input from the lateral geniculate nucleus (LGN), is further divided into 4 layers, labelled 4A, 4B, 4Cα, and 4Cβ. Sublamina 4Cα receives mostly magnocellular input from the LGN, while layer 4Cβ receives input from parvocellular pathways [11].
The average number of neurons in the adult human primary visual cortex in each hemisphere has been estimated at around 140 million.[12]
Function
V1 has a very well-defined map of the spatial information in vision. For example, in humans, the upper bank of the calcarine sulcus responds strongly to the lower half of visual field (below the center), and the lower bank of the calcarine to the upper half of visual field. In concept, this retinotopic mapping is a transformation of the visual image from retina to V1. The correspondence between a given location in V1 and in the subjective visual field is very precise: even the blind spots are mapped into V1. In terms of evolution, this correspondence is very basic and found in most animals that possess a V1. In humans and animals with a fovea in the retina, a large portion of V1 is mapped to the small, central portion of visual field, a phenomenon known as cortical magnification.[13] Perhaps for the purpose of accurate spatial encoding, neurons in V1 have the smallest receptive field size of any visual cortex microscopic regions.
The tuning properties of V1 neurons (what the neurons respond to) differ greatly over time. Early in time (40 ms and further) individual V1 neurons have strong tuning to a small set of stimuli. That is, the neuronal responses can discriminate small changes in visual orientations, spatial frequencies and colors. Furthermore, individual V1 neurons in humans and animals with binocular vision have ocular dominance, namely tuning to one of the two eyes. In V1, and primary sensory cortex in general, neurons with similar tuning properties tend to cluster together as cortical columns. David Hubel and Torsten Wiesel proposed the classic ice-cube organization model of cortical columns for two tuning properties: ocular dominance and orientation. However, this model cannot accommodate the color, spatial frequency and many other features to which neurons are tuned . The exact organization of all these cortical columns within V1 remains a hot topic of current research. The mathematical modeling of this function has been compared to Gabor transforms.
Later in time (after 100 ms), neurons in V1 are also sensitive to the more global organisation of the scene (Lamme & Roelfsema, 2000).[14] These response properties probably stem from recurrent feedback processing (the influence of higher-tier cortical areas on lower-tier cortical areas) and lateral connections from pyramidal neurons (Hupe et al. 1998). While feedforward connections are mainly driving, feedback connections are mostly modulatory in their effects (Angelucci et al., 2003; Hupe et al., 2001). Evidence shows that feedback originating in higher-level areas such as V4, IT, or MT, with bigger and more complex receptive fields, can modify and shape V1 responses, accounting for contextual or extra-classical receptive field effects (Guo et al., 2007; Huang et al., 2007; Sillito et al., 2006).
The visual information relayed to V1 is not coded in terms of spatial (or optical) imagery but rather are better described as edge detection. As an example, for an image comprising half side black and half side white, the dividing line between black and white has strongest local contrast (that is, edge detection) and is encoded, while few neurons code the brightness information (black or white per se). As information is further relayed to subsequent visual areas, it is coded as increasingly non-local frequency/phase signals. Note that, at these early stages of cortical visual processing, spatial location of visual information is well preserved amid the local contrast encoding (edge detection).
Axiomatically determined functional models of simple cells in V1 have been determined by Lindeberg[15][16] in terms of directional derivatives of affine Gaussian kernels over the spatial domain in combination with temporal derivatives of either non-causal or time-causal scale-space kernels over the temporal domain (see axiomatic theory of receptive fields). Specifically, it has been shown that this theory both leads to predictions about receptive fields with good qualitative agreement with the biological receptive field measurements performed by DeAngelis et al.[17][18] and guarantees good theoretical properties of the mathematical receptive field model, including covariance and invariance properties under natural image transformations.[19]
Differences in size of V1 also seem to have an effect on the perception of illusions.[20]
V2
Visual area V2, or secondary visual cortex, also called prestriate cortex,[21] is the second major area in the visual cortex, and the first region within the visual association area. It receives strong feedforward connections from V1 (direct and via the pulvinar) and sends strong connections to V3, V4, and V5. It also sends strong feedback connections to V1.
In terms of anatomy, V2 is split into four quadrants, a dorsal and ventral representation in the left and the right hemispheres. Together, these four regions provide a complete map of the visual world. V2 has many properties in common with V1: Cells are tuned to simple properties such as orientation, spatial frequency, and colour. The responses of many V2 neurons are also modulated by more complex properties, such as the orientation of illusory contours,[22][23] binocular disparity,[24] and whether the stimulus is part of the figure or the ground.[25][26] Recent research has shown that V2 cells show a small amount of attentional modulation (more than V1, less than V4), are tuned for moderately complex patterns, and may be driven by multiple orientations at different subregions within a single receptive field.
It is argued that the entire ventral visual-to-hippocampal stream is important for visual memory.[27] This theory, unlike the dominant one, predicts that object-recognition memory (ORM) alterations could result from the manipulation in V2, an area that is highly interconnected within the ventral stream of visual cortices. In the monkey brain, this area receives strong feedforward connections from the primary visual cortex (V1) and sends strong projections to other secondary visual cortices (V3, V4, and V5).[28][29] Most of the neurons of this area in primates are tuned to simple visual characteristics such as orientation, spatial frequency, size, color, and shape.[23][30][31] Anatomical studies implicate layer 3 of area V2 in visual-information processing. In contrast to layer 3, layer 6 of the visual cortex is composed of many types of neurons, and their response to visual stimuli is more complex.
In a recent study, the Layer 6 cells of the V2 cortex were found to play a very important role in the storage of Object Recognition Memory as well as the conversion of short-term object memories into long-term memories.[32]
Third visual cortex, including area V3
The term third visual complex refers to the region of cortex located immediately in front of V2, which includes the region named visual area V3 in humans. The "complex" nomenclature is justified by the fact that some controversy still exists regarding the exact extent of area V3, with some researchers proposing that the cortex located in front of V2 may include two or three functional subdivisions. For example, David Van Essen and others (1986) have proposed the existence of a "dorsal V3" in the upper part of the cerebral hemisphere, which is distinct from the "ventral V3" (or ventral posterior area, VP) located in the lower part of the brain. Dorsal and ventral V3 have distinct connections with other parts of the brain, appear different in sections stained with a variety of methods, and contain neurons that respond to different combinations of visual stimulus (for example, colour-selective neurons are more common in the ventral V3). Additional subdivisions, including V3A and V3B have also been reported in humans. These subdivisions are located near dorsal V3, but do not adjoin V2.
Dorsal V3 is normally considered to be part of the dorsal stream, receiving inputs from V2 and from the primary visual area and projecting to the posterior parietal cortex. It may be anatomically located in Brodmann area 19. Braddick using fMRI has suggested that area V3/V3A may play a role in the processing of global motion[33] Other studies prefer to consider dorsal V3 as part of a larger area, named the dorsomedial area (DM), which contains a representation of the entire visual field. Neurons in area DM respond to coherent motion of large patterns covering extensive portions of the visual field (Lui and collaborators, 2006).
Ventral V3 (VP), has much weaker connections from the primary visual area, and stronger connections with the inferior temporal cortex. While earlier studies proposed that VP contained a representation of only the upper part of the visual field (above the point of fixation), more recent work indicates that this area is more extensive than previously appreciated, and like other visual areas it may contain a complete visual representation. The revised, more extensive VP is referred to as the ventrolateral posterior area (VLP) by Rosa and Tweedale.[34]
V4
Visual area V4 is one of the visual areas in the extrastriate visual cortex. In macaques, it is located anterior to V2 and posterior to posterior inferotemporal area (PIT). It comprises at least four regions (left and right V4d, left and right V4v), and some groups report that it contains rostral and caudal subdivisions as well. It is unknown whether the human V4 is as expansive as that of the macaque homologue which is a subject of debate.[35]
V4 is the third cortical area in the ventral stream, receiving strong feedforward input from V2 and sending strong connections to the PIT. It also receives direct input from V1, especially for central space. In addition, it has weaker connections to V5 and dorsal prelunate gyrus (DP).
V4 is the first area in the ventral stream to show strong attentional modulation. Most studies indicate that selective attention can change firing rates in V4 by about 20%. A seminal paper by Moran and Desimone characterizing these effects was the first paper to find attention effects anywhere in the visual cortex.[36]
Like V2, V4 is tuned for orientation, spatial frequency, and color. Unlike V2, V4 is tuned for object features of intermediate complexity, like simple geometric shapes, although no one has developed a full parametric description of the tuning space for V4. Visual area V4 is not tuned for complex objects such as faces, as areas in the inferotemporal cortex are.
The firing properties of V4 were first described by Semir Zeki in the late 1970s, who also named the area. Before that, V4 was known by its anatomical description, the prelunate gyrus. Originally, Zeki argued that the purpose of V4 was to process color information. Work in the early 1980s proved that V4 was as directly involved in form recognition as earlier cortical areas. This research supported the two-streams hypothesis, first presented by Ungerleider and Mishkin in 1982.
Recent work has shown that V4 exhibits long-term plasticity,[37] encodes stimulus salience, is gated by signals coming from the frontal eye fields,[38] and shows changes in the spatial profile of its receptive fields with attention.
Middle temporal visual area (V5)
The middle temporal visual area (MT or V5) is a region of extrastriate visual cortex. In several species of both New World monkeys and Old World monkeys the MT area contains a high concentration of direction-selective neurons.[39] The MT in primates is thought to play a major role in the perception of motion, the integration of local motion signals into global percepts, and the guidance of some eye movements.[39]
Connections
MT is connected to a wide array of cortical and subcortical brain areas. Its input comes from visual cortical areas V1, V2 and dorsal V3 (dorsomedial area),[40][41] the koniocellular regions of the LGN,[42] and the inferior pulvinar.[43] The pattern of projections to MT changes somewhat between the representations of the foveal and peripheral visual fields, with the latter receiving inputs from areas located in the midline cortex and retrosplenial region.[44]
A standard view is that V1 provides the "most important" input to MT.[39] Nonetheless, several studies have demonstrated that neurons in MT are capable of responding to visual information, often in a direction-selective manner, even after V1 has been destroyed or inactivated.[45] Moreover, research by Semir Zeki and collaborators has suggested that certain types of visual information may reach MT before it even reaches V1.
MT sends its major output to areas located in the cortex immediately surrounding it, including areas FST, MST, and V4t (middle temporal crescent). Other projections of MT target the eye movement-related areas of the frontal and parietal lobes (frontal eye field and lateral intraparietal area).
Function
The first studies of the electrophysiological properties of neurons in MT showed that a large portion of the cells are tuned to the speed and direction of moving visual stimuli.[46][47]
Lesion studies have also supported the role of MT in motion perception and eye movements.[48] Neuropsychological studies of a patient unable to see motion, seeing the world in a series of static 'frames' instead, suggested that V5 in the primate is homologous to MT in the human.[49][50]
However, since neurons in V1 are also tuned to the direction and speed of motion, these early results left open the question of precisely what MT could do that V1 could not. Much work has been carried out on this region, as it appears to integrate local visual motion signals into the global motion of complex objects.[51] For example, lesion to the V5 leads to deficits in perceiving motion and processing of complex stimuli. It contains many neurons selective for the motion of complex visual features (line ends, corners). Microstimulation of a neuron located in the V5 affects the perception of motion. For example, if one finds a neuron with preference for upward motion in a monkey's V5 and stimulates it with an electrode, then the monkey becomes more likely to report 'upward' motion when presented with stimuli containing 'left' and 'right' as well as 'upward' components.[52]
There is still much controversy over the exact form of the computations carried out in area MT[53] and some research suggests that feature motion is in fact already available at lower levels of the visual system such as V1. [54] [55]
V6
The dorsomedial area (DM) also known as V6, appears to respond to visual stimuli associated with self-motion [58] and wide-field stimulation.[59] V6, is a subdivision of the visual cortex of primates first described by John Allman and Jon Kaas in 1975.[60] V6 is located in the dorsal part of the extrastriate cortex, near the deep groove through the centre of the brain (medial longitudinal fissure), and typically also includes portions of the medial cortex, such as the parieto-occipital sulcus. DM contains a topographically organized representation of the entire field of vision.
There are similarities between the visual area V5 and V6 of the common marmoset. Both areas receive direct connections from the primary visual cortex. And both have a high myelin content, a characteristic that is usually present in brain structures involved in fast transmission of information.
For many years, it was considered that DM only existed in New World monkeys. However, more recent research has suggested that DM also exists in Old World monkeys and perhaps humans. V6 is also sometimes referred to as the parieto-occipital area (PO), although the correspondence is not exact.[61][62]
Properties
Neurons in area DM/V6 of night monkeys and common marmosets have unique response properties, including an extremely sharp selectivity for the orientation of visual contours, and preference for long, uninterrupted lines covering large parts of the visual field.[63][64]
However, in comparison with area MT, a much smaller proportion of DM cells shows selectivity for the direction of motion of visual patterns.[65] Another notable difference with area MT is that cells in DM are attuned to low spatial frequency components of an image, and respond poorly to the motion of textured patterns such as a field of random dots.[65] These response properties suggest that DM and MT may work in parallel, with the former analyzing self-motion relative to the environment, and the latter analyzing the motion of individual objects relative to the background.[65]
Recently, an area responsive to wide-angle flow fields has been identified in the human and is thought to be a homologue of macaque area V6.[66]
Pathways
The connections and response properties of cells in DM/ V6 suggest that this area is a key node in a subset of the 'dorsal stream', referred to by some as the 'dorsomedial pathway'. This pathway is likely to be important for the control of skeletomotor activity, including postural reactions and reaching movements towards objects [62] The main 'feedforward' connection of DM is to the cortex immediately rostral to it, in the interface between the occipital and parietal lobes (V6A). This region has, in turn, relatively direct connections with the regions of the frontal lobe that control arm movements, including the premotor cortex.
See also
- Cortical area
- Cortical blindness
- Feature integration theory
- List of regions in the human brain
- Retinotopy
- Visual processing
- Visual feature array
- Complex cell
References
- Mather, George. "The Visual Cortex". School of Life Sciences: University of Sussex. University of Sussex. Retrieved 6 March 2017.
- Braz, José; Pettré, Julien; Richard, Paul; Kerren, Andreas; Linsen, Lars; Battiato, Sebastiano; Imai, Francisco (February 11, 2016). "Algorithmic Optimnizations in the HMAX Model Targeted for Efficient Object Recognition". In Bitar, Ahmad W.; Mansour, Mohamad M.; Chehab, Ali (eds.). Computer Vision, Imaging and Computer Graphics Theory and Applications. Berlin, Germany: Springer. p. 377. ISBN 9783319299716.
- Ungerleider LG, Mishkin M (1982). "Two Cortical Visual Systems". In Ingle DJ, Goodale MA, Mansfield RJ (eds.). Analysis of Visual Behavior. Boston: MIT Press. pp. 549–586.
- Goodale MA, Milner AD (1992). "Separate pathways for perception and action". Trends in Neurosciences. 15 (1): 20–25. CiteSeerX 10.1.1.207.6873. doi:10.1016/0166-2236(92)90344-8. PMID 1374953.
- Aglioti S, DeSouza JF, Goodale MA (1995). "Size-contrast illusions deceive the eye but not the hand". Current Biology. 5 (6): 679–85. doi:10.1016/S0960-9822(95)00133-3. PMID 7552179.
- Franz VH, Scharnowski F, Gegenfurtner (2005). "Illusion effects on grasping are temporally constant not dynamic". Journal of Experimental Psychology: Human Perception and Performance. 31 (6): 1359–78. doi:10.1037/0096-1523.31.6.1359. PMID 16366795.CS1 maint: multiple names: authors list (link)
- Ganel T, Goodale MA (2003). "Visual control of action but not perception requires analytical processing of object shape". Nature. 426 (6967): 664–7. Bibcode:2003Natur.426..664G. doi:10.1038/nature02156. PMID 14668865.
- Ganel T, Tanzer M, Goodale MA (2008). "A double dissociation between action and perception in the context of visual illusions: opposite effects of real and illusory size". Psychological Science. 19 (3): 221–5. doi:10.1111/j.1467-9280.2008.02071.x. PMID 18315792.
- Goodale MA. (2011). "Transforming vision into action". Vision Research. 51 (14): 1567–87. doi:10.1016/j.visres.2010.07.027. PMID 20691202.
- Glickstein M., Rizzolatti G. Francesco Gennari and the structure of the cerebral cortex Trends in Neurosciences, Volume 7, Issue 12, 464–467, 1 December 1984.
- Hubel, D.H., Wiesel, T.N.. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. Journal of Comparative Neurology, Issue 146, 421–450, 1972.
- Leuba G; Kraftsik R (1994). "Changes in volume, surface estimate, three-dimensional shape and total number of neurons of the human primary visual cortex from midgestation until old age". Anatomy and Embryology. 190 (4): 351–366. doi:10.1007/BF00187293. PMID 7840422.
- Barghout, Lauren (1999). On the Differences Between Peripheral and Foveal Pattern Masking. Berkeley, California, U.S.A.: Masters Thesis. U.C. Berkeley.
- Barghout, Lauren (2003). Vision: How Global Perceptual Context Changes Local Contrast Processing (Ph.D. Dissertation). Updated to include computer vision techniques. Scholar's Press. ISBN 978-3-639-70962-9.
- Lindeberg, T. (2013). "A computational theory of visual receptive fields". Biological Cybernetics. 107 (6): 589–635. doi:10.1007/s00422-013-0569-z. PMC 3840297. PMID 24197240.
- Lindeberg, T. (2016). "Time-causal and time-recursive spatio-temporal receptive fields". Journal of Mathematical Imaging and Vision. 55 (1): 50–88. arXiv:1504.02648. doi:10.1007/s10851-015-0613-9.
- DeAngelis, G. C.; Ohzawa, I.; Freeman, R. D. (1995). "Receptive field dynamics in the central visual pathways". Trends in Neurosciences. 18 (10): 451–457. doi:10.1016/0166-2236(95)94496-r. PMID 8545912.
- G. C. DeAngelis and A. Anzai "A modern view of the classical receptive field: linear and non-linear spatio-temporal processing by V1 neurons. In: Chalupa, L.M., Werner, J.S. (eds.) The Visual Neurosciences, vol. 1, pp. 704–719. MIT Press, Cambridge, 2004.
- Lindeberg, T. (2013). "Invariance of visual operations at the level of receptive fields". PLOS ONE. 8 (7): e66990. arXiv:1210.0754. Bibcode:2013PLoSO...866990L. doi:10.1371/journal.pone.0066990. PMC 3716821. PMID 23894283.
- Schwarzkopf, D Samuel (2011). "The surface area of human V1 predicts the subjective experience of object size". Nature Neuroscience. 14 (1): 28–30. doi:10.1038/nn.2706. PMC 3012031. PMID 21131954.
- Gazzaniga, Ivry & Mangun: Cognitive neuroscience, 2002
- von der Heydt, R; Peterhans, E; Baumgartner, G (1984). "Illusory contours and cortical neuron responses". Science. 224 (4654): 1260–62. Bibcode:1984Sci...224.1260V. doi:10.1126/science.6539501. PMID 6539501.
- Anzai, A; Peng, X; Van Essen, D. C (2007). "Neurons in monkey visual area V2 encode combinations of orientations". Nature Neuroscience. 10 (10): 1313–21. doi:10.1038/nn1975. PMID 17873872.
- von der Heydt, R; Zhou, H; Friedman, H. S (2000). "Representation of stereoscopic edges in monkey visual cortex". Vision Research. 40 (15): 1955–67. doi:10.1016/s0042-6989(00)00044-4. PMID 10828464.
- Qiu, F. T; von der Heydt, R (2005). "Figure and ground in the visual cortex: V2 combines stereoscopic cues with Gestalt rules". Neuron. 47 (1): 155–66. doi:10.1016/j.neuron.2005.05.028. PMC 1564069. PMID 15996555.
- Maruko, I; et alt. (2008). "Postnatal Development of Disparity Sensitivity in Visual Area 2 (V2) of Macaque Monkeys". Journal of Neurophysiology. 100 (5): 2486–2495. doi:10.1152/jn.90397.2008. PMC 2585398. PMID 18753321.CS1 maint: multiple names: authors list (link)
- Bussey, T J; Saksida, L. M (2007). "Memory, perception, and the ventral visual-perirhinal-hippocampal stream: thinking outside of the boxes". Hippocampus. 17 (9): 898–908. doi:10.1002/hipo.20320. PMID 17636546.
- Stepniewska, I; Kaas, J. H. (1996). "Topographic patterns of V2 cortical connections in macaque monkeys". The Journal of Comparative Neurology. 371 (1): 129–152. doi:10.1002/(SICI)1096-9861(19960715)371:1<129::AID-CNE8>3.0.CO;2-5. PMID 8835723.
- Gattas, R; Sousa, A. P; Mishkin, M; Ungerleider, L. G. (1997). "Cortical projections of area V2 in the macaque". Cerebral Cortex. 7 (2): 110–129. doi:10.1093/cercor/7.2.110. PMID 9087820.
- Hegdé, Jay; Van Essen, D. C (2000). "Selectivity for Complex Shapes in Primate Visual Area V2". The Journal of Neuroscience. 20.
- Hegdé, Jay; Van Essen, D. C (2004). "Temporal dynamics of shape analysis in Macaque visual area V2". Journal of Psychology. 92 (5): 3030–3042. doi:10.1152/jn.00822.2003. PMID 15201315.
- López-Aranda et alt. (2009). "Role of Layer 6 of V2 Visual Cortex in Object Recognition Memory". Science. 325 (5936): 87–89. Bibcode:2009Sci...325...87L. doi:10.1126/science.1170869. PMID 19574389.
- Braddick, OJ, O'Brien, JM; et al. (2001). "Brain areas sensitive to coherent visual motion". Perception. 30 (1): 61–72. doi:10.1068/p3048. PMID 11257978.CS1 maint: multiple names: authors list (link)
- Rosa MG, Tweedale R (2000). "Visual areas in lateral and ventral extrastriate cortices of the marmoset monkey". Journal of Comparative Neurology. 422 (4): 621–51. doi:10.1002/1096-9861(20000710)422:4<621::AID-CNE10>3.0.CO;2-E. PMID 10861530.
- Goddard E; Goddard, Erin; McDonald, J. S.; Solomon, S. G.; Clifford, C. W. G.; et al. (2011). "Color responsiveness argues against a dorsal component of human V4". Journal of Vision. 11 (4): 3. doi:10.1167/11.4.3. PMID 21467155.
- Moran, J; Desimone, R (1985). "Selective Attention Gates Visual Processing in the Extrastriate Cortex". Science. 229 (4715): 782–4. Bibcode:1985Sci...229..782M. CiteSeerX 10.1.1.308.6038. doi:10.1126/science.4023713. PMID 4023713.
- Schmid, M. C.; Schmiedt, J. T.; Peters, A. J.; Saunders, R. C.; Maier, A.; Leopold, D. A. (27 November 2013). "Motion-Sensitive Responses in Visual Area V4 in the Absence of Primary Visual Cortex" (PDF). Journal of Neuroscience. 33 (48): 18740–18745. doi:10.1523/JNEUROSCI.3923-13.2013. PMC 3841445. PMID 24285880.
- Moore, Tirin; Armstrong, Katherine M. (2003). "Selective gating of visual signals by microstimulation of frontal cortex". Nature. 421 (6921): 370–373. Bibcode:2003Natur.421..370M. doi:10.1038/nature01341. PMID 12540901.
- Born R, Bradley D (2005). "Structure and function of visual area MT". Annual Review of Neuroscience. 28: 157–89. doi:10.1146/annurev.neuro.26.041002.131052. PMID 16022593.
- Felleman D, Van Essen D (1991). "Distributed hierarchical processing in the primate cerebral cortex". Cerebral Cortex. 1 (1): 1–47. doi:10.1093/cercor/1.1.1-a. PMID 1822724.
- Ungerleider L, Desimone R (1986). "Cortical connections of visual area MT in the macaque". Journal of Comparative Neurology. 248 (2): 190–222. doi:10.1002/cne.902480204. PMID 3722458.
- Sincich L, Park K, Wohlgemuth M, Horton J (2004). "Bypassing V1: a direct geniculate input to area MT". Nature Neuroscience. 7 (10): 1123–8. doi:10.1038/nn1318. PMID 15378066.
- Warner CE, Goldshmit Y, Bourne JA (2010). "Retinal afferents synapse with relay cells targeting the middle temporal area in the pulvinar and lateral geniculate nuclei". Frontiers in Neuroanatomy. 4: 8. doi:10.3389/neuro.05.008.2010. PMC 2826187. PMID 20179789.
- Palmer SM, Rosa MG (2006). "A distinct anatomical network of cortical areas for analysis of motion in far peripheral vision". European Journal of Neuroscience. 24 (8): 2389–405. doi:10.1111/j.1460-9568.2006.05113.x. PMID 17042793.
- Rodman HR, Gross CG, Albright TD (1989). "Afferent basis of visual response properties in area MT of the macaque. I. Effects of striate cortex removal". Journal of Neuroscience. 9 (6): 2033–50. doi:10.1523/JNEUROSCI.09-06-02033.1989. PMC 6569731. PMID 2723765.
- Dubner R, Zeki S (1971). "Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey". Brain Research. 35 (2): 528–32. doi:10.1016/0006-8993(71)90494-X. PMID 5002708..
- Maunsell J, Van Essen D (1983). "Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation". Journal of Neurophysiology. 49 (5): 1127–47. doi:10.1152/jn.1983.49.5.1127. PMID 6864242.
- Dursteler M.R.; Wurtz R.H.; Newsome W.T. (1987). "Directional pursuit deficits following lesions of the foveal representation within the superior temporal sulcus of the macaque monkey". Journal of Neurophysiology. 57 (5): 1262–87. CiteSeerX 10.1.1.375.8659. doi:10.1152/jn.1987.57.5.1262. PMID 3585468.
- Hess R.H.; Baker C.L.; Zihl J. (1989). "The 'motion-blind' patient: low-level spatial and temporal filters". Journal of Neuroscience. 9 (5): 1628–40. doi:10.1523/JNEUROSCI.09-05-01628.1989. PMC 6569833. PMID 2723744.
- Baker C.L. Jr; Hess R.F; Zihl J. (1991). "Residual motion perception in a 'motion-blind' patient, assessed with limited-lifetime random dot stimuli". Journal of Neuroscience. 11 (2): 454–61. doi:10.1523/JNEUROSCI.11-02-00454.1991. PMC 6575225. PMID 1992012.
- Movshon, J.A., Adelson, E.H., Gizzi, M.S., & Newsome, W.T. (1985). The analysis of moving visual patterns. In: C. Chagas, R. Gattass, & C. Gross (Eds.), Pattern recognition mechanisms (pp. 117–151), Rome: Vatican Press.
- Britten K.H.; van Wezel R.J. (1998). "Electrical microstimulation of cortical area MST biases heading perception in monkeys". Nature Neuroscience. 1 (1): 59–63. doi:10.1038/259. PMID 10195110.
- Wilson, H.R.; Ferrera, V.P.; Yo, C. (1992). "A psychophysically motivated model for two-dimensional motion perception". Visual Neuroscience. 9 (1): 79–97. doi:10.1017/s0952523800006386. PMID 1633129.
- Tinsley, C.J., Webb, B.S., Barraclough, N.E., Vincent, C.J., Parker, A., & Derrington, A.M. (2003). "The nature of V1 neural responses to 2D moving patterns depends on receptive-field structure in the marmoset monkey". Journal of Neurophysiology. 90 (2): 930–7. doi:10.1152/jn.00708.2002. PMID 12711710.CS1 maint: multiple names: authors list (link)
- Pack C.C.; Born R.T.; Livingstone M.S. (2003). "Two-dimensional substructure of stereo and motion interactions in macaque visual cortex". Neuron. 37 (3): 525–35. doi:10.1016/s0896-6273(02)01187-x. PMID 12575958.
- Albright T (1984). "Direction and orientation selectivity of neurons in visual area MT of the macaque". Journal of Neurophysiology. 52 (6): 1106–30. doi:10.1152/jn.1984.52.6.1106. PMID 6520628.
- DeAngelis G, Newsome W (1999). "Organization of disparity-selective neurons in macaque area MT". Journal of Neuroscience. 19 (4): 1398–415. doi:10.1523/JNEUROSCI.19-04-01398.1999. PMC 6786027. PMID 9952417.
- Cardin, V; Smith, AT (2010). "Sensitivity of human visual and vestibular cortical regions to stereoscopic depth gradients associated with self-motion". Cerebral Cortex. 20 (8): 1964–73. doi:10.1093/cercor/bhp268. PMC 2901022. PMID 20034998.
- Pitzalis et alt. (2006). "Wide-Field Retinotopy Defines Human Cortical Visual Area V6". The Journal of Neuroscience. 26 (30): 7962–73. doi:10.1523/jneurosci.0178-06.2006. PMC 6674231. PMID 16870741.
- Allman JM, Kaas JH (1975). "The dorsomedial cortical visual area: a third tier area in the occipital lobe of the owl monkey (Aotus trivirgatus)". Brain Research. 100 (3): 473–487. doi:10.1016/0006-8993(75)90153-5. PMID 811327.
- Galletti C, et al. (2005). "The relationship between V6 and PO in macaque extrastriate cortex" (PDF). European Journal of Neuroscience. 21 (4): 959–970. CiteSeerX 10.1.1.508.5602. doi:10.1111/j.1460-9568.2005.03911.x. PMID 15787702.
- Galletti C, et al. (2003). "Role of the medial parieto-occipital cortex in the control of reaching and grasping movements". Experimental Brain Research. 153 (2): 158–170. doi:10.1007/s00221-003-1589-z. PMID 14517595.
- Baker JF, et al. (1981). "Visual response properties of neurons in four extrastriate visual areas of the owl monkey (Aotus trivirgatus): a quantitative comparison of medial, dorsomedial, dorsolateral, and middle temporal areas". Journal of Neurophysiology. 45 (3): 397–416. doi:10.1152/jn.1981.45.3.397. PMID 7218008.
- Lui LL, et al. (2006). "Functional response properties of neurons in the dorsomedial visual area of New World monkeys (Callithrix jacchus)". Cerebral Cortex. 16 (2): 162–177. doi:10.1093/cercor/bhi094. PMID 15858163.
- http://www.fmritools.com/kdb/grey-matter/occipital-lobe/calcarine-visual-cortex/index.html
- Pitzalis, S., Sereno, M.I., Committeri, G., Fattori, P., Galati, G., Patria, F., & Galletti, C. (2010). "Human v6: The medial motion area". Cerebral Cortex. 20 (2): 411–424. doi:10.1093/cercor/bhp112. PMC 2803738. PMID 19502476.CS1 maint: uses authors parameter (link)
External links
| Wikimedia Commons has media related to Visual cortex. |
- The Primary Visual Cortex by Matthew Schmolesky at University of Utah
- Architecture of the Visual Cortex, by David Hubel at Harvard University
- ancil-415 at NeuroNames – striate area 17
- ancil-699 at NeuroNames – Brodmann area 17 in guenon
- Stained brain slice images which include the "visual%20cortex" at the BrainMaps project
- Simulator for computational modeling of visual cortex maps at topographica.org