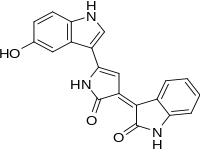
Violacein
Violacein is a naturally-occurring bis-indole pigment with antibiotic (anti-bacterial, anti-viral, anti-fungal and anti-tumor) properties.[1][2][3][4] Violacein occurs in several species of bacteria and accounts for their striking purple hues. Violacein shows increasing commercially interesting uses, especially for industrial applications in cosmetics, medicines and fabrics.
 | |
| Names | |
|---|---|
| IUPAC name
(3E)-3-[5-(5-Hydroxy-1H-indol-3-yl)-2-oxo-1,2-dihydro-3H-pyrrol-3-ylidene]-1,3-dihydro-2H-indol-2-one | |
| Other names
3-(2-(5-Hydroxyindol-3-yl)-5-oxo-2-pyrrolin-4-ylidene)-2-indolinone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
SMILES
| |
| Properties | |
Chemical formula |
C20H13N3O3 |
| Molar mass | 343.342 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis
Violacein is formed by enzymatic condensation of two tryptophan molecules, requiring the action of five proteins. The genes required for its production, vioABCDE, and the regulatory mechanisms employed have been studied within a small number of violacein-producing strains.[2]
Antibiotic activity
Violacein is known to have diverse biological activities, including as a cytotoxic anticancer agent and antibacterial action against Staphylococcus aureus and other gram-positive pathogens.[1][3][5][6] Determining the biological roles of this pigmented molecule has been of particular interest to researchers, and understanding violacein's function and mechanism of action is relevant to potential applications. Commercial production of violacein and related compounds has proven difficult so improving fermentative yields of violacein is being pursued through genetic engineering and synthetic biology.[2]
References
- Durán, Nelson; Justo, Giselle Z.; Durán, Marcela; Brocchi, Marcelo; Cordi, Livia; Tasic, Ljubica; Castro, Guillermo R.; Nakazato, Gerson (2016). "Advances in Chromobacterium violaceum and properties of violacein-Its main secondary metabolite: A review". Biotechnology Advances. 34 (5): 1030–1045. doi:10.1016/j.biotechadv.2016.06.003. PMID 27288924.
- Myeong, Nu Ri; Seong, Hoon Je; Kim, Hye-Jin; Sul, Woo Jun (2016). "Complete genome sequence of antibiotic and anticancer agent violacein producing Massilia sp. Strain NR 4-1". Journal of Biotechnology. 223: 36–7. doi:10.1016/j.jbiotec.2016.02.027. PMID 26916415.
- Choi, Seong Yeol; Yoon, Kyoung-hye; Lee, Jin Il; Mitchell, Robert J. (2015). "Violacein: Properties and Production of a Versatile Bacterial Pigment". BioMed Research International. 2015: 1–8. doi:10.1155/2015/465056. PMC 4538413. PMID 26339614.
- Andrighetti-Fröhner, C. R.; Antonio, R. V.; Creczynski-Pasa, T. B.; Barardi, C. R. M.; Simões, C. M. O. (September 2003). "Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum". Memórias do Instituto Oswaldo Cruz. 98 (6): 843–848. doi:10.1590/s0074-02762003000600023. ISSN 0074-0276. PMID 14595466.
- Lichstein, H. C.; Van De Sand, V. F. (1946). "The Antibiotic Activity of Violacein, Prodigiosin, and Phthiocol". Journal of Bacteriology. 52 (1): 145–6. PMC 518152. PMID 16561146.
- Lichstein, H., & Van De Sand, V. (1945). "Violacein, an Antibiotic Pigment Produced by Chromobacterium violaceum". Journal of Infectious Diseases. 76 (1): 47–51. doi:10.1093/infdis/76.1.47. JSTOR 30085685.CS1 maint: multiple names: authors list (link)