Vesicular monoamine transporter
The vesicular monoamine transporter (VMAT) is a transport protein integrated into the membrane of synaptic vesicles of presynaptic neurons. It acts to transport monoamine neurotransmitters – such as dopamine, serotonin, norepinephrine, epinephrine, and histamine – into the vesicles, which release the neurotransmitters into synapses as chemical messages to postsynaptic neurons. VMATs utilize a proton gradient generated by V-ATPases in vesicle membranes to power monoamine import.
Pharmaceutical drugs that target VMATs have possible applications for many conditions, leading to a plethora of biological research. These applications include hypertension, drug addiction, psychiatric disorders, Parkinson's disease, and other neurological disorders. Many drugs that target VMAT act as inhibitors and alter the kinetics of the protein. Much research regarding the effects of altered VMATs on biological systems is still ongoing.
Monoamines
Monoamines transported by VMATs are mainly noradrenaline, adrenaline, dopamine, serotonin, histamine, and trace amines.[1] Exogenous substrates include guanethidine and MPP+.[2]
Discovery
VMAT research began in 1958 with the discovery of secretory vesicles by Nils-Åke Hillarp. In the 1970s, scientists such as Arvid Carlsson recognized the need to understand how transport systems and ion gradients work in different organisms in order to explore new treatment options such as reserpine. Researchers discovered inhibitors that blocked the uptake of neurotransmitters into vesicles, suggesting the existence of VMATs.[3] A decade later molecular genetic tools have improved methods for protein identification. Scientists have used these tools to analyze DNA and amino acid sequences, discovering that transporters in bacteria and humans were very similar. This finding illustrated the importance and universality of transporters.[4] The transporters were first structurally identified by cloning VMATs in rats.[3] VMAT was first isolated and purified in bovine chromaffin granules, in both native and denatured forms.[5]
Location
There are two types of VMATs expressed in humans: VMAT1 and VMAT2.[4] VMAT1 is expressed mainly in large dense-core vesicles (LDCVs) of the peripheral nervous system. VMAT1 may be found in neuroendocrine cells, particularly chromaffin and enterochromaffin granules which are largely found in the medulla of the adrenal glands.
VMAT2 favors expression in a variety of monoaminergic cells of the CNS such as the brain, sympathetic nervous system, mast cells, and cells containing histamine in the gut. It is also prevalent in β-cells of the pancreas.[6] It is also expressed blood platelets.[7][8]
VMAT2 is also co-expressed in chromaffin cells.[6] Expression of the two transporters in internal organs seems to differ between species: only VMAT1 is expressed in the rat adrenal medulla cells whereas VMAT2 is the major transporter in the bovine adrenal medulla cells.[9]
Structure and Function
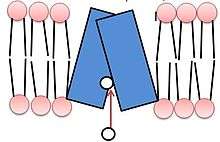
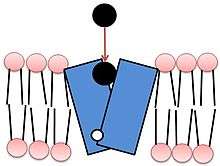
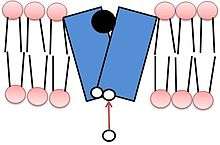
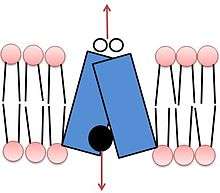
Both isoforms of VMAT, VMAT1 and VMAT2, are acidic glycoproteins with a molecular weight of approximately 70 kDa.[4][10] Both isoforms are transmembrane proteins with 12 transmembrane domains (TMD’s).[4]
VMAT functions in loading the neurotransmitters dopamine, serotonin, histamine, norepinephrine, and epinephrine into transport vesicles.[11] Collectively these neurotransmitters are referred to as monoamines. VMAT uses the same transport mechanism for all types of monoamines.[5] VMATs transport monoamines from the cytosol into high-concentration storage vesicles.[4] Transport vesicles are released into the space between neurons, called the synaptic cleft, where they convey a chemical message to the next neuron. VMATs also function in sorting, storing, and releasing neurotransmitters, and are believed to participate in protecting these neurotransmitters from autoxidation.[4] VMATs are also known to continue biochemical modification after loading of certain neurotransmitters.[4]
Vesicle packing requires a large energy source to store large numbers of neurotransmitters into a small vesicular space at high concentrations. VMAT transport relies upon the pH and electrochemical gradient generated by a vesicular H+-ATPase for this energy source.[4][12] The current model of VMAT function proposes that efflux of two protons against the H+ gradient is coupled with influx of one monoamine.[4][12] The first H+ efflux generates a transporter conformation associated with a high-affinity amine-binding site in the cytosolic phase; the second H+ efflux is coupled with a second large conformational change that leads to amine transport from the cytosolic side into the vesicle, reducing amine-binding affinity.[4]
Studies indicate that the amino acid residue His419, located on the domain between TMD X and XI of rat VMAT1, plays a role in energy coupling to the amine transport by assisting the first proton-dependent conformational change.[4][13] It has been proposed that Reserpine (RES) inhibits VMAT by interacting with this conformation.
VMAT gene sequence analysis demonstrates that 4 aspartic acid residues in the middle region of TMD I, VI, X, and XI and one Lysine residue in TMDII have highly conserved gene sequences, suggesting these residues play a critical role in transporter structure and function.[4][14] Specifically, the residues Lys 139 and Asp 427 are thought to compose an ion pair that promotes high-affinity interaction with VMAT substrates and inhibitors.[4][14] The Asp431 residue located in TMD XI is believed to be critical for amine transport, but does not interact with RES binding; this residue is thought to complete the substrate transport cycle.[4][15]
Kinetics
VMATs have a relatively low Vmax, with an estimated rate of 5–20/sec depending on the substrate.[16] Vesicle filling may limit monoamine release from neurons with high rates of firing.
Specific amine-binding affinity varies by VMAT isoform; studies indicate that catecholamines dopamine, norepinephrine, and epinephrine have three-fold higher affinity for VMAT2 binding than for VMAT1 binding and uptake.[4][12][17] The Imidazoleamine Histamine has a thirty-fold higher affinity for VMAT2 compared to VMAT1[4] and is thought to bind to a different site from that of other monoamines.[12] Unlike catecholamines and histamine, the indoleamine serotonin (5HT) binds to VMAT1 and VMAT2 with a similar affinity for both transporter isoforms.[4][17]
VMAT1 has a lower turnover number and a lower affinity for most monoamine substrates than VMAT2. This may be because of VMAT2's location in the central nervous system, which demands fast recovery from neurotransmitter release in order to prepare for the subsequent release. The uptake efficiencies of each VMAT substrate can be ranked in order of efficiency as follows: serotonin, dopamine, epinephrine, and norepinephrine.[4]
Methamphetamines decrease Vmax, while cocaine increases Vmax reversibly in rat brain.[4]
Inhibition
The effects of VMAT inhibition have been studied in-depth in animal models. Mutant homozygous VMAT(-/-) mice move little, feed poorly, and die within a few days of birth.
More specifically, inhibition of VMAT2 may cause an increase in cytosolic catecholamine levels. This can result in an increase in efflux of catecholamines through the plasma membrane, depleting catecholamine concentrations and causing increased oxidative stress and oxidative damage to the neuron.
Heterozygous VMAT mutants display hypersensitivity to amphetamine, cocaine and MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), the latter being a substance causally linked to Parkinson's disease in rodents.[7] This suggests a protective role of VMATs against oxidative stress through removal of such substances from the cytosol.[7]
VMAT inhibitors include:
- Reserpine(RES), bietaserpine, and ketanserin(KET) (potent inhibitors of VMAT2 mediated serotonin transport)
- Tetrabenazine(TBZ) (specific to VMAT2)
- Phenylethylamine
- Amphetamine
- MDMA
- N-methyl-4-phenylpyridinium (MPP+)(very potent inhibitors of VMAT2 mediated serotonin transport)
- Fenfluramine (specific to VMAT1 )
- Non-hydrolysable GTP-analogue guanylyllimidodiphosphate GMP-P(NH)P (VMAT2 only)
Binding Site Structures
Ligand-binding affinities and structures
Two known binding sites for VMAT inhibitors include the Reserpine (RES) binding site and the Tetrabenazine (TBZ) binding site. Some evidence suggests these two sites may overlap or may actually exist as two separate conformations of the same binding site.[4][12] VMAT inhibitors tend to fall into two classes; those that interact with the RES binding site and those that interact with the TBZ binding site.[12]
Reserpine (RES), Methoxytetrabenazine (MTBZ), and the drug Amiodarone bind to the RES binding site conformation. Tetrabenazine (TBZ, also called Nitoman and Xenazine), Dihydrotetrabenazine (DTBZOH), Ketanserin (KET), and the drug Lobeline bind to the TBZ binding site/conformation. Amphetamine, methamphetamine and GZ-7931 are also known to interact with VMAT2.[4][18][19][20]
Inhibitor affinity varies among VMAT isoforms. RES and KET have higher inhibitory affinity for VMAT2–mediated 5HT transport than for that of VMAT1; TBZ seems to inhibit VMAT2 exclusively.[4]
The residues aspartate-33 and serines-180, 181, and 182 are believed to be involved in substrate recognition; these residues interact with the protonated amino group and hydroxyl group on the catechol or indole rings.[12]
Cocaine and methylphenidate (MPD, also known as Ritalin and Concerta) are believed to interact with VMAT2 in such a way that causes a shift in VMAT2 protein "from a plasmalemmal membrane-associated fraction to a vesicle-enriched, nonmembrane-associated fraction."[21]
Reserpine binding site
Consistent with catecholamine-binding affinity, Reserpine (RES) has a threefold higher affinity for VMAT2 than for VMAT1.[12][17] The RES binding site is known to be hydrophobic, and this is thought to contribute to ligand binding affinity.[4] Methamphetamine binds to the reserpine site on VMATs.[22]
The current working model proposes that RES and the substrate bind to a single site in a pH-gradient modulated conformational structure of the transporter. This conformation occurs after the transport of one proton across the membrane and into the vesicle; proton transport drives the substrate recognition site from the lumen to the cytoplasmic surface of the vesicle for RES and substrate binding.[4][12][23] Methoxytetrabenazine (MTBZ) may bind to the RES binding site, based on studies indicating that RES significantly inhibited MTBZ-binding.[4] The drug Amiodarone is also believed to inhibit monoamine vesicular uptake by binding to the RES binding site.[4]
Tetrabenazine binding site
Tetrabenazine (TBZ) and Dihydrotetrabenazine (DTBZOH) are believed to bind to a different binding site from the RES/substrate binding site, or to a different conformation of the RES/substrate binding site.[4][12][24] This site is believed to be located at the N-terminus, based on studies done in bovine VMAT2.[12] Tyrosine-434 and aspartate-461 are identified as being responsible for the high-affinity interaction of TBZ, serotonin, and histamine in VMAT2.[12] Unlike methamphetamine, amphetamine binds to the TBZ site on hVMAT2.[22]
Unlike Reserpine inhibition, TBZ inhibition is affected only by very high concentrations of monoamines; however, single injections of Reserpine can inhibit TBZ binding.[12] Ketanserin (KET)[4][23] and the drug Lobeline[4][12] also bind to the TBZ binding site conformation.
Glycosolation sites: N- and C-linked terminals
Three to four glycosolation sites exist in the vesicular matrix on a loop between TMDI and TMDII.[4] In biology, the vesicle matrix refers to the material or tissue between cells in which more specialized structures are embedded. Two of the glycosylation sites, the N-linked glycosylation terminal and C-linked terminal, are located in the cytosolic portion of the vesicle.[4][25]
The highest amount of genetic variance between VMAT1 and VMAT2 exists near the N- and C- terminals in the cytosolic phase, and in the glycosylated loop between transmembrane domains I and II.[4]
C-Terminus and VMAT Trafficking Cycle
Several motifs involved in the VMAT trafficking cycle are believed to be encoded in the C-terminus. A dileucine motif in the C-terminus is required for VMAT2 endocytosis.[6] Studies suggest the acidic residues in the dileucine motif sort VMAT2 away from constitutive secretory vesicles and into the regulated secretory pathway.[6] The hydrophobic residues in the dileucine motif are thought to further couple with the acidic residues as a single unit to help sort VMAT2 to large dense course vesicles.[6] Acidic glutamate residues located upstream of the dileucine motif are known to be important for localization of VMAT2 to large dense core vesicles; these residues are also conserved in VMAT1.[6]
Genetic expression and transporter regulation
Although both VMAT1 and VMAT2 are encoded by two different genes, the individual genetic sequences demonstrate high homology.Polymorphisms in VMAT2 that effect regulation and quantitative expression may pose genetic risk factors for Parkinson's disease. Moreover, a specific VMAT1 gene (SLC18A1) has several associated polymorphisms which have a locus 8p21.3 that has been strongly connected to schizophrenia susceptibility.[26]
Over-expression of VMAT2 results in increased secretion of neurotransmitter upon cell stimulation. Data suggests that deletion of the VMAT2 genes does not affect the size of small clear-core vesicles.
VMATs may be regulated by changes in transcription, post-transcriptional modifications such as phosphorylation and mRNA splicing of exons, and vesicular transport inactivation facilitated by heterotrimeric G-proteins. It is thought that chromaffin granules possess these heterotrimeric G-proteins which have shown to be regulatory to small clear-core vesicles.[6][7]
Specific heterotrimeric G-protein type regulation is tissue-dependent for VMAT2; it is not known whether this is the case for VMAT1. Heterotrimeric G-protein Gαo2 decreases VMAT1 activity in pancreatic and adrenal medulla cells, and activates heterotrimeric G-proteins inhibit VMAT2 activity in the brain, regardless of whether localised on small clear-core or large-dense-core vesicles. Activated heterotrimeric G-protein Gαq downregulates VMAT2 mediated serotonin transport in blood platelets, but this is not the case in the brain where Gαq inhibits VMAT2 activity completely.[7] Although the exact signalling pathway for G-protein mediated regulation of VMATs is not known,[7] it has recently been described that implicated G-proteins act directly on the VMATs themselves.[27]
Clinical significance
VMAT2 has been shown to contribute to many clinical neurological disorders including drug addiction, mood disorders and stress,[28] as well as Parkinson's Disease[29] and Alzheimer's Disease.[30][31]
Parkinson's disease
Studies indicate VMAT2 mRNA is present in all cell groups damaged by Parkinson's Disease (PD);[32] these findings have identified VMAT2 as a target for Parkinson's prevention. VMAT2 presence does not independently protect neurons from Parkinsonian damage; however, a decrease in VMAT2 expression has been shown to correlate with susceptibility to Parkinson's Disease[32] and this may be due to a ratio between the Dopamine transporter and VMAT2.[32]
Based on the understanding the increased cytosolic Dopamine levels lead to dopaminergic cell death in PD, it has been proposed that regulatory polymorphisms in VMAT2 affect VMAT2 quantitative expression and may serve as a genetic risk factor for PD. Specifically, the SLC18A2 promoter region for the VMAT2 gene has been identified as an area where several polymorphisms form discrete haplotypes.[4][33]
Mood disorders
Studies using a genetic rodent model to understand clinical depression in humans suggest that VMAT2 genetic or functional alterations may play a role in depression.[34] Reduced VMAT2 levels were identified in specific subregions of the striatum involved in clinical depression, including the nucleus accumbens shell but not the core, the ventral tegmental area, and the substantia nigra pars compacta. The reduced VMAT2 protein levels were not accompanied by similar levels of VMAT2 mRNA alterations. Based on these findings it has been proposed that VMAT2 activity is not altered at the level of genetic expression, but may rather be altered at the functional level in ways that may correlate with clinical depression.[4]
Drug addiction
Many Psychostimulant drugs are known to interact with VMAT, including Amphetamine analogs such as Methamphetamine (METH), Cocaine, and Ecstasy (MDMA). See the Pharmacology section of this article for more information on these drugs' interactions.
Pharmacology
As addressed above, VMAT inhibitors tend to fall into two classes; those that interact with the RES binding site and those that interact with the TBZ binding site.[12]
Reserpine, methoxytetrabenazine, and the drug amiodarone bind to the RES binding site conformation.
Tetrabenazine (branded Nitoman and Xenazine), dihydrotetrabenazine, ketanserin, and the drug lobeline bind to the TBZ binding site/conformation.
Substituted amphetamines, including but not limited to methamphetamine, as well as cocaine, are known to interact with VMAT2. Studies indicate that both amphetamines and cocaine act to increase non-exocytotic release of dopamine in specific regions of the brain by interacting directly with VMAT2 function.[4][18][21]
Methamphetamine
VMAT is a main target of methamphetamine. Studies indicate that substituted amphetamines including methamphetamine interact with VMAT2 at the TBZ/DTBZOH binding site/conformation.[4][21] By acting as a competitive antagonist, methamphetamine blocks the presynaptic cell's ability to use VMAT for vesicular packaging.
Methamphetamine alters the subcellular location of VMAT2 which affects the distribution of dopamine in the cell. Treatment with methamphetamine relocates VMAT2 from a vesicle-enriched fraction to a location that is not continuous with synaptosomal preparations.[9]
Repeated amphetamine exposure may increase VMAT2 mRNA in certain brain regions with little or no decline upon withdrawal from the drug.[9]
A study performed by Sonsalla et al. demonstrates that methamphetamine treatment decreases DHTBZ binding and vesicular Dopamine uptake.[4][21] Another study demonstrated that multiple high doses of methamphetamine removed DTBZ binding sites from the vesicles.[12]
In addition to an interaction with the TBZ/DTBZOH binding site, some propose that substituted amphetamines like methamphetamine decrease dopamine uptake because of the weak base properties of substituted amphetamines.[21] This “Weak Base Hypothesis” proposes that amphetamine analogs enters the cell through transport and lipophilic diffusion then likewise diffuses through the vesicular membrane where it accumulates in synaptic vesicles and offsets the proton electrochemical gradient in the vesicle that drives monoamine transport through VMAT.[21] In this way, amphetamine administration would prevent vesicular DA uptake through VMAT, and explain the finding that amphetamine administration correlates with decreased dopamine release from vesicles and a neurotoxic increase in intracellular dopamine.[4][21]
Cocaine
Unlike methamphetamine, the psychostimulant cocaine interacts with VMAT2 in such a way that mobilizes VMAT2-expressing vesicles, causing a shift in VMAT2 protein from a plasmalemmal (synaptosomal) membrane fraction to a vesicle-enriched fraction that is not associated with the synaptosomal membrane and not retained in synaptosomal preparations.[4][12][21] The drug methylphenidate (branded Ritalin and Concerta) is believed to interact with VMAT2 in a similar fashion.[21]
In addition to mobilizing VMAT2-expressing vesicles, cocaine has been shown to increase Vmax of VMAT2 for dopamine, and to increase the number of DTBZ binding sites.[12] Cocaine has also been shown to mobilize a synapsin-dependent reserve pool of dopamine-containing synaptic vesicles, thereby interacting with the vesicular trafficking cycle to increase dopamine release.[12]
Short-term exposure to cocaine increases VMAT2 density in the prefrontal cortex and striatum of mammalian brains. This is theorised to be a defensive mechanism against the depletive effects cocaine has on cytosolic dopamine through increasing monoamine storage capacity.[9] Chronic cocaine use has been implicated with a reduction in VMAT2 immunoreactivity as well as a decrease in DTBZOH binding in humans.
Research suggests a decline in VMAT2 protein through prolonged cocaine use could play an important role in the development of cocaine induced mood-disorders.[9]
MDMA
The psychostimulant MDMA (popularized as ecstasy or XTC) is known to affect serotonergic neurons, but has been shown to inhibit synaptosomal and vesicular uptake of serotonin and dopamine[4] to roughly the same extent in vitro.[12] in vivo studies indicate short-term MDMA exposure causes short-term reduction in VMAT2 activity, which is reversed after 24h.[12]
Current Research
Clinical Research
Genetic research models have shown that polymorphisms in SLC18A1 and SLC18A2, the genes that encode for VMAT1 and 2 proteins respectively, may confer risk for some neuropsychiatric disorders;.[4][33][35] however, no specific diseases have yet been identified as directly resulting from a genetic mutation in an SLC18 gene, the gene that codes for VMAT proteins.[35]
Much of the current research related to VMAT explores the genetic underpinnings of neuropsychiatric disorders as they may be affected by SLC18A family mutations.
The dopaminergic neuron is known to play a central role in drug addiction and abuse and the potential role of the dopamine transporter (DAT) has been well-explored as a target for amphetamine and cocaine. Current research looks toward VMAT2 as a target for such psychostimulants. This is discussed in the Pharmacology section of this article. A combination of imaging, neurochemical, biochemical, cell biological, genetic, and immunohistochemical evidence has been compiled to provide the most current comprehensive understanding of the role the VMAT2 plays in AMPH and cocaine abuse and addiction through aminergic neurotransmission.[1][35]
As VMATs are membrane proteins, structural information is limited and researchers have yet to completely understand the structure of both isoforms. Further studies are needed in order to determine the structure and therefore complete function of these proteins. There is preliminary evidence that the gene for VMAT1 may be linked to susceptibility to schizophrenia, bipolar disorder, and various anxiety disorders.[4] Further studies are needed in order to confirm these findings and to gain a better understanding of the role of VMATs in the central nervous system.
Multiple single-nucleotide polymorphisms (SNPs) have been identified in the coding region of VMATs. The effects of some of these SNPs have been alteration of VMAT function, structure and regulation.[36] Further investigation of these SNPs is required in order to distinguish whether they may be attributable to certain diseases with suspected SNP-mutation origins.
α-synuclein, a cytosolic protein found mainly in pre-synaptic nerve terminals has been found to have regulatory interactions with the trafficking of VMATs. Moreover, mutations involving α-synuclein have been linked to familial Parkinson’s Disease.[36] Further research is needed to clarify the extent to which these proteins modulate the trafficking of VMATs and whether they may be exploited in order to gather more information as to the exact mechanism of how disorders such as Parkinson’s occur, and therefore, how they may potentially be treated.
Studies have shown that at the synaptic membrane, enzymes responsible for the synthesis of dopamine, tyrosine hydroxylase (TH) and amino acid aromatic decarboxylase (AADC) are physically and functionally coupled with VMAT2.[36] It was initially thought that the synthesis of these substances and the subsequent packaging of them into vesicles were two entirely separate processes. Such a finding could impact the approach to treatment methods for dopamine-related disorders such as schizophrenia and Parkinson’s Disease.
Animal research
Current research related to VMAT uses VMAT2 knockout mice to explore the behavioral genetics of this transporter in an animal model. VMAT2 knockouts are known to be lethal as homozygotes, but heterozygote knockouts are not lethal and are used in many studies as a durable animal model.[35] For more complete discussions on VMAT2 animal research, see discussions in the following review articles: (Lawal & Krantz, 2013),[9][35]
From knockout and knockdown mice researchers have discovered that it is good to have over-expression or under-expression of the VMAT genes in some circumstances.[35] Mice are also used in drug studies, particularity studies involving the effect cocaine and methamphetamine have on VMATs.[35] Studies involving animals have prompted scientists to work on developing drugs that inhibit or enhance the function of VMATs. Drugs that inhibit VMATs may have use in addiction but further studies are needed.[35] Enhancing the function of VMATs may also have therapeutic value.[35]
References
- Eiden LE, Weihe E (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Ann. N. Y. Acad. Sci. 1216: 86–98. doi:10.1111/j.1749-6632.2010.05906.x. PMC 4183197. PMID 21272013.
VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) ... [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC).
- Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. p. 167. ISBN 978-0-443-07145-4.
- Eiden L.; Schäfer M. H.; et al. (2004). "The vesicular amine transporter family (SLC18) amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine". Pflügers Archiv. 447 (5): 636–640. doi:10.1007/s00424-003-1100-5. PMID 12827358.
- Wimalasena K (2011). "Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry". Med Res Rev. 31 (4): 483–519. doi:10.1002/med.20187. PMC 3019297. PMID 20135628.
- Henry J. P.; Botton D.; et al. (1994). "Biochemistry and molecular biology of the vesicular monoamine transporter from chromaffin granules". J Exp Biol. 196: 251–62. PMID 7823026.
- Fei H.; Grygoruk A.; et al. (2008). "Trafficking of vesicular neurotransmitter transporters". Traffic. 9 (9): 1425–36. doi:10.1111/j.1600-0854.2008.00771.x. PMC 2897747. PMID 18507811.
- Brunk I.; Höltje B.; et al. (2006). Regulation of vesicular monoamine and glutamate transporters by vesicle-associated trimeric G-proteins: new jobs for long-known signal transduction molecules. Handbook of Experimental Pharmacology. 175. pp. 305–25. doi:10.1007/3-540-29784-7_15. ISBN 978-3-540-29783-3. PMID 16722242.
- Höltje M.; Winter S.; et al. (May 2003). "The vesicular monoamine content regulates VMAT2 activity through Galphaq in mouse platelets. Evidence for autoregulation of vesicular transmitter uptake". Journal of Biological Chemistry. 278 (18): 15850–15858. doi:10.1074/jbc.M212816200. PMID 12604601.
- Wimalasena K (2010). "Vesicular monoamine transporters: structure-function, pharmacology and medicinal chemistry". Medicinal Research Reviews. 31 (4): 483–19. doi:10.1002/med.20187. PMC 3019297. PMID 20135628.
- Liu Y, Peter D, Rogahani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH (1992). "A cDNA that suppresses MPP1 toxicity encodes a vesicular amine transporter". Cell. 70 (4): 539–551. doi:10.1016/0092-8674(92)90425-c. PMID 1505023.
- Purves, Dale, et al. Neuroscience. Sinauer Associates. 087893646
- Chaudhry FA, Edwards RH, Fonnum F (2007). "Vesicular neurotransmitter transporters as targets for endogenous and exogenous toxic substances". Annu. Rev. Pharmacol. Toxicol. 48: 277–301. doi:10.1146/annurev.pharmtox.46.120604.141146. PMID 17883368.
- Shirvan A, Laskar O, Steiner-Mordoch S, Schuldiner S. (1994). "Histidine-419 plays a role in energy coupling in the vesicular monoamine transporter from rat." FEBS Lett',' 356:145–150.
- Merickel A, Kaback HR, Edwards RH (1997). "Charged residues in transmembrane domains II and XI of a vesicular monoamine transporter form a charge pair that promotes high affinity substrate recognition". J. Biol. Chem. 272 (9): 5403–5408. doi:10.1074/jbc.272.9.5403. PMID 9038139.
- Steiner-Mordoch S, Shirvan A, Schuldiner S (1996). "Modification of the pH profile and tetrabenazine sensitivity of rat VMAT1 by replacement of aspartate 404 with glutamate". J. Biol. Chem. 271 (22): 13048–13054. doi:10.1074/jbc.271.22.13048. PMID 8662678.
- Peter D; et al. (1994). "The chromaffin granule and synaptic vesicle amine transporters differ in substrate recognition and sensitivity to inhibitors". J Biol Chem. 269: 7231–7237.
- Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E (1996). "Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter". Proc. Natl. Acad. Sci. USA. 93 (10): 5166–5171. doi:10.1073/pnas.93.10.5166. PMC 39426. PMID 8643547.
- Miller GW, Gainetdinov RR, Levey AI, Caron MG (1999). "Dopamine transporters and neuronal injury". Trends in Pharmacological Sciences. 20 (10): 424–429. doi:10.1016/S0165-6147(99)01379-6. PMID 10498956.
- Fleckenstein AE, Volz TJ, Hanson GR (2009). "Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: Neurotoxic and therapeutic implications". Neuropharmacology. 56 (Suppl 1): 133–138. doi:10.1016/j.neuropharm.2008.07.002. PMC 2634813. PMID 18662707.
- Vesicular monoamine transporter 2#Binding sites and ligands
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR (2007). "New insights into the mechanism of action of amphetamines". Annu Rev Pharmacol Toxicol. 47: 681–98. doi:10.1146/annurev.pharmtox.47.120505.105140. PMID 17209801.
- Sulzer D, Sonders MS, Poulsen NW, Galli A (April 2005). "Mechanisms of neurotransmitter release by amphetamines: a review". Prog. Neurobiol. 75 (6): 406–433. doi:10.1016/j.pneurobio.2005.04.003. PMID 15955613.
They also demonstrated competition for binding between METH and reserpine, suggesting they might bind to the same site on VMAT. George Uhl's laboratory similarly reported that AMPH displaced the VMAT2 blocker tetrabenazine (Gonzalez et al., 1994). It should be noted that tetrabenazine and reserpine are thought to bind to different sites on VMAT (Schuldiner et al., 1993a)
- Darchen F, Scherman D, Henry JP (1989). "Reserpine binding to chromaffin granules suggests the existence of two conformations of the monoamine transporter". Biochemistry. 28 (4): 1692–1697. doi:10.1021/bi00430a040. PMID 2719928.
- Liu Y, Edwards RH (1997). "The role of vesicular transport proteins in synaptic transmission and neural degeneration". Annu. Rev. Neurosci. 20: 125–156. doi:10.1146/annurev.neuro.20.1.125. PMID 9056710.
- Erickson, Eiden, & Hoffman, 1992
- Lohoff, Falk W.; Weller, Andrew E.; Bloch, Paul J.; Buono, Russell J.; Doyle, Glenn A.; Ferraro, Thomas N.; Berrettini, Wade H. (2008). "Association between Polymorphisms in the Vesicular Monoamine Transporter 1 Gene (VMAT1/SLC18A1) on Chromosome 8p and Schizophrenia". Neuropsychobiology. 57 (1–2): 55–60. doi:10.1159/000129668. ISSN 0302-282X. PMID 18451639.
- Remin, R., Schuldiner, S., 2003. Vesicular neurotransmitter transporters: Pharmacology, Biochemistry and Molecular Analysis. Neurotransmitter Transporters; Structure, Function, and Regulation, pp.313-354
- Tillinger A, Sollas A, Serova LI, Kvetnansky R, Sabban EL (2010). "Vesicular monoamine transporters (VMATs) in adrenal chromaffin cells: stress-triggered induction of VMAT2 and expression in epinephrine synthesizing cells". Cell Mol Neurobiol. 30 (8): 1459–1465. doi:10.1007/s10571-010-9575z (inactive 2019-12-04).
- Okamura N, Villemagne VL, Drago J, Pejoska S, Dhamija RK, Mulligan RS, Ellis JR, Ackermann U, O'Keefe G, Jones G, Kung HF, Pontecorvo MJ, Skovronsky D, Rowe CC (2010). "In vivo measurement of vesicular monoamine transporter type 2 density in Parkinson disease with (18)F-AV-133". J. Nucl. Med. 51 (2): 223–228. doi:10.2967/jnumed.109.070094. PMID 20080893.
- Villemagne VL, Okamura N, Pejoska S, Drago J, Mulligan RS, Chetelat G, Ackermann U, O'Keefe G, Jones G, Gong S, Tochon-Danguy H, Kung HF, Masters CL, Skovronsky DM, Rowe CC (2011). "In vivo assessment of vesicular monoamine transporter type 2 in dementia with lewy bodies and Alzheimer disease". Arch. Neurol. 68 (7): 905–912. doi:10.1001/archneurol.2011.142. PMID 21747030.
- Salin A, Savli M, Lanzenberger R (2011). "Serotonin and molecular neuroimaging in humans using PET". Amino Acids. 42 (6): 2039–57. doi:10.1007/s00726-011-1078-9. PMID 21947614.
- Miller GW, Gainetdinov RR, Levey AI, Caron MG (1999). "Dopamine transporters and neuronal injury". TiPS. 20: 425.
- Glatt CE, Wahner AD, White DJ, Ruiz-Linares A, Ritz B (2006). "Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women". Hum. Mol. Genet. 15 (2): 299–305. doi:10.1093/hmg/ddi445. PMC 3643966. PMID 16339215.
- Schwartz K, Yadid G, Weizman A, Rehavi M (2003). "Decreased limbic vesicular monoamine transporter 2 in a genetic rat model of depression". Brain Res. 965 (1–2): 174–179. doi:10.1016/s0006-8993(02)04167-7. PMID 12591135.
- Lawal HO, Krantz DE (2013). "SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine". Molecular Aspects of Medicine. 34 (2–3): 360–372. doi:10.1016/j.mam.2012.07.005. PMC 3727660. PMID 23506877.
- Sager, J.J. & Torres, G.E., 2011. Proteins interacting with monoamine transporters: Current state and future challenges. Biochemistry, [online] Available at: <http://pubs.acs.org/doi/ipdf/10.1021/bi200405c>[Accessed 20 April 2013]
External links
- Vesicular+Monoamine+Transport+Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
Further reading
- Kilbourn MR (1997). "In vivo radiotracers for vesicular neurotransmitter transporters". Nucl. Med. Biol. 24 (7): 615–9. doi:10.1016/S0969-8051(97)00101-7. PMID 9352531.
- Lawal HO, Krantz DE (2013). "SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine". Molecular Aspects of Medicine. 34 (2–3): 360–372. doi:10.1016/j.mam.2012.07.005. PMC 3727660. PMID 23506877.
- Weihe E, Eiden LE (2000). "Chemical neuroanatomy of the vesicular amine transporters". FASEB J. 14 (15): 2435–49. CiteSeerX 10.1.1.334.4881. doi:10.1096/fj.00-0202rev. PMID 11099461.
- Wimalasena, K. (2011). "Vesicular Monoamine Transporters: Structure-Function, Pharmacology, and Medicinal Chemistry". Medicinal Research Reviews. 31 (4): 483–519. doi:10.1002/med.20187. PMC 3019297. PMID 20135628.