Tezacaftor
Tezacaftor, development code VX-661, is a drug that is sold as a fixed-dose combination with ivacaftor as Symdeko. It is used for the treatment of cystic fibrosis (CF) in people six years and older, who have specific gene mutations.[1][2]
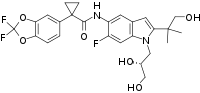 | |
| Clinical data | |
|---|---|
| Trade names | Symdeko (with ivacaftor) |
| Other names | VX-661 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H27F3N2O6 |
| Molar mass | 520.505 g·mol−1 |
InChI
| |
In 2018, the U.S. Food and Drug Administration (FDA) approved a combination of ivacaftor and tezacaftor;[1] the manufacturer announced a list price of $292,000 per year.[3] The combination of ivacaftor and tezacaftor is being sold as Symdeko.[1][4]
Mechanism of action
Tezacaftor helps move the cystic fibrosis transmembrane conductance regulator (CFTR) protein to the correct position on the cell surface, and is designed to treat people with the F508del mutation.[1][5]
Clinical trials
The EVOLVE and EXPAND study findings were published in 2017.[4]
History
The U.S. Food and Drug Administration (FDA) granted the application priority review and granted the approval of Symdeko to Vertex Pharmaceuticals Incorporated.[2]
References
- "Drug Trials Snapshots: Symdeko". U.S. Food and Drug Administration. 7 March 2018. Archived from the original on 21 November 2019. Retrieved 20 November 2019.

- "FDA expands approval of treatment for cystic fibrosis to include patients ages 6 and older". U.S. Food and Drug Administration (FDA) (Press release). 21 June 2019. Archived from the original on 21 November 2019. Retrieved 20 November 2019.

- "FDA approves another Vertex drug for treatment of cystic fibrosis". The Boston Globe. 17 July 2018. Retrieved 20 November 2019.
- FDA Approves New Cystic Fibrosis Drug Combo - Symdeko approved for patients with specific CFTR mutations Feb 2018
- Tezacaftor (VX-661) for Cystic Fibrosis
External links
- "Tezacaftor". Drug Information Portal. U.S. National Library of Medicine.
- "Ivacaftor regimen with Tezacaftor". Drug Information Portal. U.S. National Library of Medicine.