Tenatoprazole
Tenatoprazole is a proton pump inhibitor drug candidate that was undergoing clinical testing as a potential treatment for reflux oesophagitis and peptic ulcer as far back as 2003.[1] The compound was invented by Mitsubishi Tanabe Pharma and was licensed to Negma Laboratories (part of Wockhardt as of 2007[2]).[3]:22
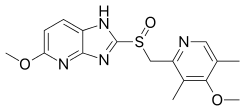 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP2C19-mediated) |
| Elimination half-life | 4.8 to 7.7 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.120.697 |
| Chemical and physical data | |
| Formula | C16H18N4O3S |
| Molar mass | 346.405 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| (verify) | |
Mitsubishi reported that tenatoprazole was still in Phase I clinical trials in 2007[4]:27 and again in 2012.[3]:17
Tenatoprazole has an imidazopyridine ring in place of the benzimidazole moiety found in other proton pump inhibitors, and has a half-life about seven times longer than other PPIs.[5]
See also
References
- "Gastrointestinal Disease Update". Digestive Disease Week. DataMonitor. March 2003.
- "Investors unwilling to forgive Wockhardt, promoter for failings". Economic Times. 3 March 2011.
- "State of New Product Development" (PDF). Mitsubishi Tanabe Pharma. 8 May 2012.
- "FY2007 Interim Financial Results". Mitsubishi Tanabe Pharma.
- Li H, Meng L, Liu F, Wei JF, Wang YQ (January 2013). "H+/K+-ATPase inhibitors: a patent review". Expert Opinion on Therapeutic Patents. 23 (1): 99–111. doi:10.1517/13543776.2013.741121. PMID 23205582.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.