Starling equation
The Starling equation for fluid filtration is named for the British physiologist Ernest Starling, who is also recognised for the Frank–Starling law of the heart. The classic Starling equation has in recent years been revised. The Starling principle of fluid exchange is key to understanding how plasma fluid (solvent) within the bloodstream (intravascular fluid) moves to the space outside the bloodstream (extravascular space). Starling can be credited with identifying that the "absorption of isotonic salt solutions (from the extravascular space) by the blood vessels is determined by this osmotic pressure of the serum proteins." (1896)
Transendothelial fluid exchange occurs predominantly in the capillaries, and is a process of plasma ultrafiltration across a semi-permeable membrane. It is now appreciated that the ultrafilter is the endothelial glycocalyx layer whose interpolymer spaces function as a system of small pores, radius circa 5 nm. Where the endothelial glycocalyx overlies an inter endothelial cell cleft, the plasma ultrafiltrate may pass to the interstitial space. Some continuous capillaries may feature fenestrations that provide an additional subglycocalyx pathway for solvent and small solutes. Discontinuous capillaries as found in sinusoidal tissues of bone marrow, liver and spleen have little or no filter function.[1]
The rate at which fluid is filtered across vascular endothelium (transendothelial filtration) is determined by the sum of two outward forces, capillary pressure () and interstitial protein osmotic pressure (), and two absorptive forces, plasma protein osmotic pressure () and interstitial pressure (). The Starling equation describes these forces in mathematical terms. It is one of the Kedem–Katchalski equations which bring nonsteady state thermodynamics to the theory of osmotic pressure across membranes that are at least partly permeable to the solute responsible for the osmotic pressure difference (Staverman 1951; Kedem and Katchalsky 1958). The second Kedem–Katchalsky equation explains the trans endothelial transport of solutes, .
Glomerular capillaries have a continuous glycocalyx layer in health and the total transendothelial filtration rate of solvent () to the renal tubules is normally around 125 ml/ min (about 180 litres/ day). Glomerular capillary is more familiarly known as the glomerular filtration rate (GFR). In the rest of the body's capillaries, is typically 5 ml/ min (around 8 litres/ day), and the fluid is returned to the circulation via afferent and efferent lymphatics.
The equation
The classic Starling equation reads as follows:
where:
- is the trans endothelial solvent filtration volume per second (SI units of m3·s−1).
- is the net driving force (SI units of Pa = kg·m−1·s−2, often expressed as mmHg),
- is the capillary hydrostatic pressure
- is the interstitial hydrostatic pressure
- is the plasma protein oncotic pressure
- is the interstitial oncotic pressure
- is the hydraulic conductivity of the membrane (SI units of m2·s·kg−1, equivalent to m·s−1·mmHg−1)
- is the surface area for filtration (SI units of m2)
- the product · is defined as the filtration coefficient (SI units of m4·s·kg−1, or equivalently in m3·s−1·mmHg−1)
- is Staverman's reflection coefficient (adimensional)
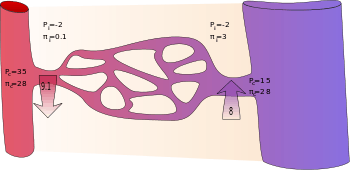
By convention, outward force is defined as positive, and inward force is defined as negative. If Jv is positive, solvent is leaving the capillary (filtration). If negative, solvent is entering the capillary (absorption). Applying the classic Starling equation, it has long been believed and taught that continuous capillaries filter fluid in their arteriolar section and reabsorb most of it in their venular section, as shown by the diagram. In fact, in most tissues and most of the time continuous capillaries are in a state of filtration along their entire length, and filtered fluid is mostly returned to the circulation via lymph nodes and the thoracic duct.[2] The mechanism for this "no reabsorption rule" is called the glycocalyx model, or the Michel-Weinbaum model in honour of two scientists who independently described the glycocalyx model. Briefly, the interstitial fluid colloid osmotic pressure πi has been found to have no effect on Jv and the colloid osmotic pressure difference that opposes filtration is now known to be π'p minus the subglycocalyx π, which is close to zero while there is adequate filtration to flush interstitial proteins out of the inter endothelial cleft. Consequently, Jv is much less than previously calculated, and the unopposed diffusion of interstitial proteins to the subglycocalix space if and when filtration falls wipes out the colloid osmotic pressure difference necessary for reabsorption of fluid to the capillary.
The revised Starling equation is compatible with the steady-state Starling principle:
where:
- is the trans endothelial solvent filtration volume per second.
- is the net driving force,
- is the capillary hydrostatic pressure
- is the interstitial hydrostatic pressure
- is the plasma protein oncotic pressure
- is the subglycocalyx oncotic pressure
- is the hydraulic conductivity of the membrane
- is the surface area for filtration
- is Staverman's reflection coefficient
Pressures are often measured in millimetres of mercury (mmHg), and the filtration coefficient in millilitres per minute per millimetre of mercury (ml·min−1·mmHg−1).
Filtration coefficient
In some texts the product of hydraulic conductivity and surface area is called the filtration co-efficient Kfc.
Reflection coefficient
Staverman's reflection coefficient (σ) corrects the actual colloid osmotic pressure difference to the observed or effective pressure. Since the discovery of the endothelial glycocalyx layer it is now helpful to think of σ as indicative of the effectiveness of the glycocalyx ultrafilter. Where σ is close to 1 the glycocalyx layer is fully effective in allowing solvent and smaller solutes to filter to the extravascular space while larger molecules such as albumin and other plasma proteins are retained. Where σ is much less than 1.0, the glycocalyx filter function is reduced.
- Glomerular capillaries have a reflection coefficient close to 1 as normally no protein crosses into the glomerular filtrate.
- In contrast, hepatic sinusoids have no reflection coefficient as they are fully permeable to protein. Hepatic interstitial fluid within the Space of Diss has the same colloid osmotic pressure as plasma and so hepatocyte synthesis of albumin can be regulated. Albumin and other proteins in the interstitial spaces return to the circulation via lymph.
Approximated values
Following are typically quoted values for the variables in the classic Starling equation:
| Location | Pc (mmHg)[3] | Pi (mmHg)[3] | σπc (mmHg)[3] | σπi (mmHg)[3] |
|---|---|---|---|---|
| arteriolar end of capillary | +35 | −2 | +28 | +0.1 |
| venular end of capillary | +15 | −2 | +28 | +3 |
It is reasoned that some albumin escapes from the capillaries and enters the interstitial fluid where it would produce a flow of water equivalent to that produced by a hydrostatic pressure of +3 mmHg. Thus, the difference in protein concentration would produce a flow of fluid into the vessel at the venous end equivalent to 28 − 3 = 25 mmHg of hydrostatic pressure. The total oncotic pressure present at the venous end could be considered as +25 mmHg.
In the beginning (arteriolar end) of a capillary, there is a net driving force () outwards from the capillary of +9 mmHg. In the end (venular end), on the other hand, there is a net driving force of −8 mmHg.
Assuming that the net driving force declines linearly, then there is a mean net driving force outwards from the capillary as a whole, which also results in that more fluid exits a capillary than re-enters it. The lymphatic system drains this excess.
J. Rodney Levick argues in his textbook that the interstitial force is often underestimated, and measurements used to populate the revised Starling equation show the absorbing forces to be consistently less than capillary or venular pressures.
Clinical usefulness
The principles behind the equation are considered useful for explaining physiological phenomena happening at the capillary such as the formation of edema.
Woodcock and Woodcock showed in 2012 that the revised Starling equation (steady-state Starling principle) provides scientific explanations for clinical observations concerning intravenous fluid therapy.[4]
See also
References
- Levick, J (2010). Introduction to Cardiovascular Physiology. 5th edition. London: Hodder Arnold. p. 190. ISBN 978-0340-942-048.
- Levick, J.R.; Michel, C.C. (2010). "Microvascular fluid exchange and the revised Starling principle". Cardiovasc Res. 87 (2): 198–210. doi:10.1093/cvr/cvq062. PMID 20200043.
- Boron, Walter F. (2005). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. ISBN 978-1-4160-2328-9.
- Woodcock, T. E.; Woodcock, T. M. (29 January 2012). "Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy". British Journal of Anaesthesia. 108 (3): 384–394. doi:10.1093/bja/aer515. PMID 22290457.