Siponimod
Siponimod, marketed under the trade name Mayzent, is a selective sphingosine-1-phosphate receptor modulator for oral use that is used for multiple sclerosis (MS). It is intended for once-daily oral administration.[2]
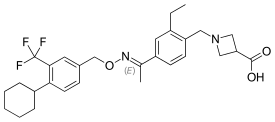 | |
| Clinical data | |
|---|---|
| Trade names | Mayzent[1] |
| Other names | BAF-312 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619027 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C29H35F3N2O3 |
| Molar mass | 516.26 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
In March 2019, it was approved in the United States to treat adults with relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.[1]
Medical uses
Siponimod was studied for the treatment of secondary progressive multiple sclerosis (SPMS), which is the progressive neurological decline of multiple sclerosis that happens independent of acute relapses.[1] In active SPMS, siponimod decreases the risk of disability and MS relapses.[1]
Adverse effects
In clinical trials of siponimod, the most common adverse effects were headache, high blood pressure, and liver function test abnormalities.[1]
Pharmacology
Mechanism of action
Siponimod binds selectively to some of the sphingosine-1-phosphate receptor forms—including sphingosine-1-phosphate receptor 1—found on lymphocytes and other cell types.
This binding inhibits the migration of the lymphocytes to the location of the inflammation (e.g. in MS).
Siponimod may be very similar to fingolimod but preventing lymphopenia, one of its main side effects, by preventing egress of lymphocytes from lymph nodes. Siponimod may be more selective in the particular sphingosine-1-phosphate receptors (five in number) that it modulates.[3] It is selective for the -1 and -5 SIP receptors.[2]
History
In March 2019, siponimod was approved in the United States to treat adults with relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.[1][4]
The efficacy of siponimod was shown in a clinical trial (Trial 1/NCT01665144) of 1,651 patients that compared siponimod to placebo in people with secondary progressive multiple sclerosis (SPMS) who had evidence of disability progression in the prior two years and no relapses in the three months prior to enrollment.[1][4] The primary endpoint of the study was the time to three-month confirmed progression in disability.[1] The trial was conducted at 294 centers in Asia, Australia, Canada, Europe, South America, and the United States.[4]
The U.S. Food and Drug Administration (FDA) granted approval of Mayzent to Novartis.[1][4]
References
- "FDA approves new oral drug to treat multiple sclerosis". U.S. Food and Drug Administration (FDA). 26 March 2019. Archived from the original on 27 November 2019. Retrieved 24 November 2019.

- "Siponimod (BAF312) for the Treatment of Secondary Progressive Multiple Sclerosis: Design of the Phase 3 EXPAND Trial (P07.126)". Archived from the original on 23 June 2015. Retrieved 6 January 2016.
- WO 2008000419, Hiestand, Peter C; Schnell, Christian, "S1P Receptor modulators for treating multiple sclerosis", assigned to Novartis
- "Drug Trials Snapshots: Mayzent". U.S. Food and Drug Administration (FDA). 19 April 2019. Archived from the original on 28 September 2019. Retrieved 24 November 2019.

External links
- "Siponimod". Drug Information Portal. U.S. National Library of Medicine.
- "Drug Approval Package: Mayzent (siponimod)". U.S. Food and Drug Administration (FDA).