Sarcoidosis
Sarcoidosis is a disease involving abnormal collections of inflammatory cells that form lumps known as granulomas.[2] The disease usually begins in the lungs, skin, or lymph nodes.[2] Less commonly affected are the eyes, liver, heart, and brain.[2] Any organ, however, can be affected.[2] The signs and symptoms depend on the organ involved.[2] Often, none, or only mild, symptoms are seen.[2] When it affects the lungs, wheezing, coughing, shortness of breath, or chest pain may occur.[3] Some may have Löfgren syndrome with fever, large lymph nodes, arthritis, and a rash known as erythema nodosum.[2]
| Sarcoidosis | |
|---|---|
| Other names | Sarcoïdosis, sarcoid, Besnier-Boeck-Schaumann disease[1] |
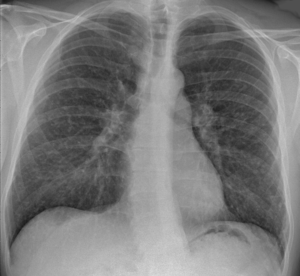 | |
| Chest X-ray showing the typical nodularity of sarcoidosis, predominantly in the bases of the lungs. | |
| Pronunciation |
|
| Specialty | Rheumatology |
| Symptoms | Depends on the organ involved[2] Lungs: wheezing, cough, shortness of breath, chest pain[3] Skin: lumps, ulcers, discolored skin[3] Children: weight loss, bone pain, feeling tired[3] |
| Usual onset | 20–50 year old women[4] |
| Duration | Few years to long term[2][5] |
| Causes | Unknown[2] |
| Risk factors | Family history[4] |
| Diagnostic method | Based on symptoms and tissue biopsy[6] |
| Differential diagnosis | Tuberculosis, lymphoma, infectious mononucleosis, pulmonary eosinophilia[7] |
| Treatment | Ibuprofen, prednisone, methotrexate[8][9] |
| Prognosis | Mortality 1–7%[5] |
| Frequency | 1.9 million with interstitial lung disease (2015)[10] |
| Deaths | 122,000 with interstitial lung disease (2015)[11] |
The cause of sarcoidosis is unknown.[2] Some believe it may be due to an immune reaction to a trigger such as an infection or chemicals in those who are genetically predisposed.[12][13] Those with affected family members are at greater risk.[4] Diagnosis is partly based on signs and symptoms, which may be supported by biopsy.[6] Findings that make it likely include large lymph nodes at the root of the lung on both sides, high blood calcium with a normal parathyroid hormone level, or elevated levels of angiotensin converting enzyme in the blood.[6] The diagnosis should only be made after excluding other possible causes of similar symptoms such as tuberculosis.[6]
Sarcoidosis may resolve without any treatment within a few years.[2][5] However, some people may have long-term or severe disease.[5] Some symptoms may be improved with the use of anti-inflammatory drugs such as ibuprofen.[8] In cases where the condition causes significant health problems, steroids such as prednisone are indicated.[9] Medications such as methotrexate, chloroquine, or azathioprine may occasionally be used in an effort to decrease the side effects of steroids.[9] The risk of death is 1-7%.[5] The chance of the disease returning in someone who has had it previously is less than 5%.[2]
In 2015, pulmonary sarcoidosis and interstitial lung disease affected 1.9 million people globally and they resulted in 122,000 deaths.[10][11] It is most common in Scandinavians, but occurs in all parts of the world.[14] In the United States, risk is greater among black as opposed to white people.[14] It usually begins between the ages of 20 and 50.[4] It occurs more often in women than men.[4] Sarcoidosis was first described in 1877 by the English doctor Jonathan Hutchinson as a nonpainful skin disease.[15]
Signs and symptoms
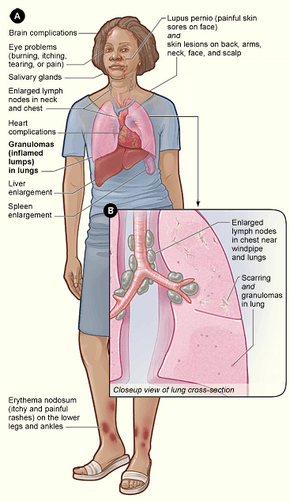
Sarcoidosis is a systemic inflammatory disease that can affect any organ, although it can be asymptomatic and is discovered by accident in about 5% of cases.[17] Common symptoms, which tend to be vague, include fatigue (unrelieved by sleep; occurs in 66% of cases), lack of energy, weight loss, joint aches and pains (which occur in about 70% of cases),[18] arthritis (14–38% of persons), dry eyes, swelling of the knees, blurry vision, shortness of breath, a dry, hacking cough, or skin lesions.[19][20][21][22] Less commonly, people may cough up blood.[19] The cutaneous symptoms vary, and range from rashes and noduli (small bumps) to erythema nodosum, granuloma annulare, or lupus pernio. Sarcoidosis and cancer may mimic one another, making the distinction difficult.[23]
The combination of erythema nodosum, bilateral hilar lymphadenopathy, and joint pain is called Löfgren syndrome, which has a relatively good prognosis.[19] This form of the disease occurs significantly more often in Scandinavian patients than in those of non-Scandinavian origin.[24]
Respiratory tract
Localization to the lungs is by far the most common manifestation of sarcoidosis.[25] At least 90% of affected persons experience lung involvement.[26] Overall, about 50% develop permanent pulmonary abnormalities, and 5 to 15% have progressive fibrosis of the lung parenchyma. Sarcoidosis of the lung is primarily an interstitial lung disease in which the inflammatory process involves the alveoli, small bronchi, and small blood vessels.[27] In acute and subacute cases, physical examination usually reveals dry crackles.[26] At least 5% of persons suffer pulmonary arterial hypertension.[26][28] The upper respiratory tract (including the larynx, pharynx, and sinuses) may be affected, which occurs in between 5 and 10% of cases.[29]
The four stages of pulmonary involvement are based on radiological stage of the disease, which is helpful in prognosis:[30]
- Stage I: bilateral hilar lymphadenopathy (BHL) alone
- Stage II: BHL with pulmonary infiltrates
- Stage III: pulmonary infiltrates without BHL
- Stage IV: fibrosis
Use of the Scadding scale only provides general information regarding the prognosis of the pulmonary disease over time. Caution is recommended, as it only shows a general relation with physiological markers of the disease and the variation is such that it has limited applicability in individual assessments, including treatment decisions.[12]
Skin
Sarcoidosis involves the skin in between 9 and 37% of persons and is more common in African Americans than in European Americans.[26] The skin is the second-most commonly affected organ after the lungs.[31] The most common lesions are erythema nodosum, plaques, maculopapular eruptions, subcutaneous nodules, and lupus pernio.[31] Treatment is not required, since the lesions usually resolve spontaneously in 2–4 weeks. Although it may be disfiguring, cutaneous sarcoidosis rarely causes major problems.[26][32][33] Sarcoidosis of the scalp presents with diffuse or patchy hair loss.[34][35]
Heart
The frequency of cardiac involvement varies and is significantly influenced by race; in Japan, more than 25% of persons with sarcoidosis have symptomatic cardiac involvement, whereas in the US and Europe, only about 5% of cases present with cardiac involvement.[26] Autopsy studies in the US have revealed a frequency of cardiac involvement of about 20–30%, whereas autopsy studies in Japan have shown a frequency of 60%.[21] The presentation of cardiac sarcoidosis can range from asymptomatic conduction abnormalities to fatal ventricular arrhythmia.[36][37]
Conduction abnormalities are the most common cardiac manifestations of sarcoidosis in humans and can include complete heart block.[38] Second to conduction abnormalities, in frequency, are ventricular arrhythmias, which occurs in about 23% of persons with cardiac involvement.[38] Sudden cardiac death, either due to ventricular arrhythmias or complete heart block is a rare complication of cardiac sarcoidosis.[39][40] Cardiac sarcoidosis can cause fibrosis, granuloma formation, or the accumulation of fluid in the interstitium of the heart, or a combination of the former two.[41][42] Cardiac sarcoidosis may also cause congestive heart failure when granulomas cause myocardial fibrosis and scarring.[43] Congestive heart failure affects 25-75% of persons with cardiac sarcoidosis. Pulmonary arterial hypertension occurs by two mechanisms in cardiac sarcoidosis: reduced left heart function due to granulomas weakening the heart muscle or from impaired blood flow.[44]
Eye
Eye involvement occurs in about 10–90% of cases.[21] Manifestations in the eye include uveitis, uveoparotitis, and retinal inflammation, which may result in loss of visual acuity or blindness.[45] The most common ophthalmologic manifestation of sarcoidosis is uveitis.[21][46][47] The combination of anterior uveitis, parotitis, VII cranial nerve paralysis and fever is called uveoparotid fever or Heerfordt syndrome (D86.8). Development of scleral nodule associated with sarcoidosis has been observed.[48]
Nervous system
Any of the components of the nervous system can be involved.[49] Sarcoidosis affecting the nervous system is known as neurosarcoidosis.[49] Cranial nerves are most commonly affected, accounting for about 5–30% of neurosarcoidosis cases, and peripheral facial nerve palsy, often bilateral, is the most common neurological manifestation of sarcoidosis.[49][50][51] It occurs suddenly and is usually transient. The central nervous system involvement is present in 10–25% of sarcoidosis cases.[29] Other common manifestations of neurosarcoidosis include optic nerve dysfunction, papilledema, palate dysfunction, neuroendocrine changes, hearing abnormalities, hypothalamic and pituitary abnormalities, chronic meningitis, and peripheral neuropathy.[26] Myelopathy, that is spinal cord involvement, occurs in about 16–43% of neurosarcoidosis cases and is often associated with the poorest prognosis of the neurosarcoidosis subtypes.[49] Whereas facial nerve palsies and acute meningitis due to sarcoidosis tend to have the most favourable prognosis,[49] another common finding in sarcoidosis with neurological involvement is autonomic or sensory small-fiber neuropathy.[52][53] Neuroendocrine sarcoidosis accounts for about 5–10% of neurosarcoidosis cases and can lead to diabetes insipidus, changes in menstrual cycle and hypothalamic dysfunction.[49][51] The latter can lead to changes in body temperature, mood, and prolactin (see the endocrine and exocrine section for details).[49]
Endocrine and exocrine
Prolactin is frequently increased in sarcoidosis, between 3 and 32% of cases have hyperprolactinemia[54] this frequently leads to amenorrhea, galactorrhea, or nonpuerperal mastitis in women. It also frequently causes an increase in 1,25-dihydroxy vitamin D, the active metabolite of vitamin D, which is usually hydroxylated within the kidney, but in sarcoidosis patients, hydroxylation of vitamin D can occur outside the kidneys, namely inside the immune cells found in the granulomas the condition produces. 1,25-dihydroxy vitamin D is the main cause for hypercalcemia in sarcoidosis and overproduced by sarcoid granulomata. Gamma-interferon produced by activated lymphocytes and macrophages plays a major role in the synthesis of 1 alpha, 25(OH)2D3.[55] Hypercalciuria (excessive secretion of calcium in one's urine) and hypercalcemia (an excessively high amount of calcium in the blood) are seen in <10% of individuals and likely results from the increased 1,25-dihydroxy vitamin D production.[56]
Thyroid dysfunction is seen in 4.2–4.6% of cases.[57][58]
Parotid enlargement occurs in about 5–10% of persons.[18] Bilateral involvement is the rule. The gland is usually not tender, but firm and smooth. Dry mouth can occur; other exocrine glands are affected only rarely.[26] The eyes, their glands, or the parotid glands are affected in 20–50% of cases.[59]
Gastrointestinal and genitourinary
Symptomatic gastrointestinal (GI) involvement occurs in less than 1% of persons (if one excludes the liver), and most commonly the stomach is affected, although the small or large intestine may also be affected in a small portion of cases.[18][60] Studies at autopsy have revealed GI involvement in less than 10% of people.[51] These cases would likely mimic Crohn's disease, which is a more commonly intestine-affecting granulomatous disease.[18] About 1–3% of people have evidence of pancreatic involvement at autopsy.[51] Symptomatic kidney involvement occurs in just 0.7% of cases, although evidence of kidney involvement at autopsy has been reported in up to 22% of people and occurs exclusively in cases of chronic disease.[18][21][51] Symptomatic kidney involvement is usually nephrocalcinosis, although granulomatous interstitial nephritis that presents with reduced creatinine clearance and little proteinuria is a close second.[18][51] Less commonly, the epididymis, testicles, prostate, ovaries, fallopian tubes, uterus, or the vulva may be affected, the latter may cause vulva itchiness.[21][61][62] Testicular involvement has been reported in about 5% of people at autopsy.[51][62] In males, sarcoidosis may lead to infertility.[62]
Around 70% of people have granulomas in their livers, although only in about 20–30% of cases, liver function test anomalies reflecting this fact are seen.[19][26] About 5–15% of persons exhibit hepatomegaly.[21] Only 5–30% of cases of liver involvement are symptomatic.[63] Usually, these changes reflect a cholestatic pattern and include raised levels of alkaline phosphatase (which is the most common liver function test anomaly seen in persons with sarcoidosis), while bilirubin and aminotransferases are only mildly elevated. Jaundice is rare.[18][26]
Blood
Abnormal blood tests are frequent, accounting for over 50% of cases, but are not diagnostic.[26][29] Lymphopenia is the most common blood anomaly in sarcoidosis.[26] Anemia occurs in about 20% of people with sarcoidosis.[26] Leukopenia is less common and occurs in even fewer persons but is rarely severe.[26] Thrombocytopenia and hemolytic anemia are fairly rare.[18] In the absence of splenomegaly, leukopenia may reflect bone marrow involvement, but the most common mechanism is a redistribution of blood T cells to sites of disease.[64] Other nonspecific findings include monocytosis, occurring in the majority of sarcoidosis cases,[65] increased hepatic enzymes or alkaline phosphatase. People with sarcoidosis often have immunologic anomalies like allergies to test antigens such as Candida or purified protein derivative.[59] Polyclonal hypergammaglobulinemia is also a fairly common immunologic anomaly seen in sarcoidosis.[59]
Lymphadenopathy (swollen glands) is common in sarcoidosis and occurs in 15% of cases.[22] Intrathoracic nodes are enlarged in 75 to 90% of all people; usually this involves the hilar nodes, but the paratracheal nodes are commonly involved. Peripheral lymphadenopathy is very common, particularly involving the cervical (the most common head and neck manifestation of the disease), axillary, epitrochlear, and inguinal nodes.[66] Approximately 75% of cases show microscopic involvement of the spleen, although only in about 5–10% of cases does splenomegaly appear.[18][59]
Bone, joints, and muscles
Sarcoidosis can be involved with the joints, bones, and muscles. This causes a wide variety of musculoskeletal complaints that act through different mechanisms.[67] About 5–15% of cases affect the bones, joints, or muscles.[29]
Arthritic syndromes can be categorized as acute or chronic.[67] Sarcoidosis patients suffering acute arthritis often also have bilateral hilar lymphadenopathy and erythema nodosum. These three associated syndromes often occur together in Löfgren syndrome.[67] The arthritis symptoms of Löfgren syndrome occur most frequently in the ankles, followed by the knees, wrists, elbows, and metacarpophalangeal joints.[67] Usually, true arthritis is not present, but instead, periarthritis appears as a swelling in the soft tissue around the joints that can be seen by ultrasonographic methods.[67] These joint symptoms tend to precede or occur at the same time as erythema nodosum develops.[67] Even when erythema nodosum is absent, it is believed that the combination of hilar lymphadenopathy and ankle periarthritis can be considered as a variant of Löfgren syndrome.[67] Enthesitis also occurs in about one-third of patients with acute sarcoid arthritis, mainly affecting the Achilles tendon and heels.[67] Soft-tissue swelling of the ankles can be prominent, and biopsy of this soft tissue reveals no granulomas, but does show panniculitis similar to erythema nodosum.[67]
Chronic sarcoid arthritis usually occurs in the setting of more diffuse organ involvement.[67] The ankles, knees, wrists, elbows, and hands may all be affected in the chronic form and often this presents itself in a polyarticular pattern.[67] Dactylitis similar to that seen in psoriatic arthritis, that is associated with pain, swelling, overlying skin erythema, and underlying bony changes may also occur.[67] Development of Jaccoud arthropathy (a nonerosive deformity) is very rarely seen.[67]
Bone involvement in sarcoidosis has been reported in 1–13% of cases.[51] The most frequent sites of involvement are the hands and feet, whereas the spine is less commonly affected.[67] Half of the patients with bony lesions experience pain and stiffness, whereas the other half remain asymptomatic.[67] Periostitis is rarely seen in sarcoidosis and has been found to present itself at the femoral bone.[68][69]
Cause
The exact cause of sarcoidosis is not known.[2] The current working hypothesis is, in genetically susceptible individuals, sarcoidosis is caused through alteration to the immune response after exposure to an environmental, occupational, or infectious agent.[70] Some cases may be caused by treatment with TNF inhibitors like etanercept.[71]
Genetics
The heritability of sarcoidosis varies according to ethnicity. About 20% of African Americans with sarcoidosis have a family member with the condition, whereas the same figure for European Americans is about 5%. Additionally, in African Americans, who seem to experience more severe and chronic disease, siblings and parents of sarcoidosis cases have about a 2.5-fold increased risk for developing the disease.[24] In Swedish individuals heritability was found to be 39%.[72] In this group, if a first-degree family member was affected, a person has a four-fold greater risk of being affected.[72]
Investigations of genetic susceptibility yielded many candidate genes, but only few were confirmed by further investigations and no reliable genetic markers are known. Currently, the most interesting candidate gene is BTNL2; several HLA-DR risk alleles are also being investigated.[73][74] In persistent sarcoidosis, the HLA haplotype HLA-B7-DR15 is either cooperating in disease or another gene between these two loci is associated. In nonpersistent disease, a strong genetic association exists with HLA DR3-DQ2.[75][76] Cardiac sarcoid has been connected to TNFA variants.[77]
Infectious agents
Several infectious agents appear to be significantly associated with sarcoidosis, but none of the known associations is specific enough to suggest a direct causative role.[78] The major implicated infectious agents include: mycobacteria, fungi, borrelia, and rickettsia.[79] A meta-analysis investigating the role of mycobacteria in sarcoidosis found it was present in 26.4% of cases, but they also detected a possible publication bias, so the results need further confirmation.[80][81] Mycobacterium tuberculosis catalase-peroxidase has been identified as a possible antigen catalyst of sarcoidosis.[82] The disease has also been reported by transmission via organ transplants.[83]
Autoimmune
Association of autoimmune disorders has been frequently observed. The exact mechanism of this relation is not known, but some evidence supports the hypothesis that this is a consequence of Th1 lymphokine prevalence.[57][84] Tests of delayed cutaneous hypersensitivity have been used to measure progression.[85]
Pathophysiology
Granulomatous inflammation is characterized primarily by the accumulation of macrophages and activated T-lymphocytes, with increased production of key inflammatory mediators, TNF, IFN-γ, IL-2, IL-8, IL-10, IL-12, IL-18, IL-23 and TGF-β, indicative of a Th1-mediated immune response.[79][86] Sarcoidosis has paradoxical effects on inflammatory processes; it is characterized by increased macrophage and CD4 helper T-cell activation, resulting in accelerated inflammation, but immune response to antigen challenges such as tuberculin is suppressed. This paradoxic state of simultaneous hyper- and hypoactivity is suggestive of a state of anergy. The anergy may also be responsible for the increased risk of infections and cancer.
The regulatory T-lymphocytes in the periphery of sarcoid granulomas appear to suppress IL-2 secretion, which is hypothesized to cause the state of anergy by preventing antigen-specific memory responses.[87] Schaumann bodies seen in sarcoidosis are calcium and protein inclusions inside of Langhans giant cells as part of a granuloma.
While TNF is widely believed to play an important role in the formation of granulomas (this is further supported by the finding that in animal models of mycobacterial granuloma formation inhibition of either TNF or IFN-γ production inhibits granuloma formation), sarcoidosis can and does still develop in persons being treated with TNF antagonists like etanercept.[88] B cells also likely play a role in the pathophysiology of sarcoidosis.[24] Serum levels of soluble HLA class I antigens and ACE are higher in persons with sarcoidosis.[24] Likewise the ratio of CD4/CD8 T cells in bronchoalveolar lavage is usually higher in persons with pulmonary sarcoidosis (usually >3.5), although it can be normal or even abnormally low in some cases.[24] Serum ACE levels have been found to usually correlate with total granuloma load.[79]
Cases of sarcoidosis have also been reported as part of the immune reconstitution syndrome of HIV, that is, when people receive treatment for HIV, their immune system rebounds and the result is that it starts to attack the antigens of opportunistic infections caught prior to said rebound and the resulting immune response starts to damage healthy tissue.[86]
_lymph_node_biopsy.jpg) Sarcoidosis in a lymph node
Sarcoidosis in a lymph node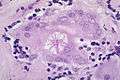 Asteroid body in sarcoidosis
Asteroid body in sarcoidosis
Diagnosis
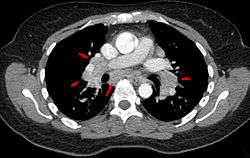
Diagnosis of sarcoidosis is a matter of exclusion, as there is no specific test for the condition. To exclude sarcoidosis in a case presenting with pulmonary symptoms might involve a chest radiograph, CT scan of chest, PET scan, CT-guided biopsy, mediastinoscopy, open lung biopsy, bronchoscopy with biopsy, endobronchial ultrasound, and endoscopic ultrasound with fine-needle aspiration of mediastinal lymph nodes (EBUS FNA). Tissue from biopsy of lymph nodes is subjected to both flow cytometry to rule out cancer and special stains (acid fast bacilli stain and Gömöri methenamine silver stain) to rule out microorganisms and fungi.[89][90][12][91]
Serum markers of sarcoidosis, include: serum amyloid A, soluble interleukin-2 receptor, lysozyme, angiotensin converting enzyme, and the glycoprotein KL-6.[92] Angiotensin-converting enzyme blood levels are used in the monitoring of sarcoidosis.[92] A bronchoalveolar lavage can show an elevated (of at least 3.5) CD4/CD8 T cell ratio, which is indicative (but not proof) of pulmonary sarcoidosis.[24] In at least one study the induced sputum ratio of CD4/CD8 and level of TNF was correlated to those in the lavage fluid.[92] A sarcoidosis-like lung disease called granulomatous–lymphocytic interstitial lung disease can be seen in patients with common variable immunodeficiency (CVID) and therefore serum antibody levels should be measured to exclude CVID.
Differential diagnosis includes metastatic disease, lymphoma, septic emboli, rheumatoid nodules, granulomatosis with polyangiitis, varicella infection, tuberculosis, and atypical infections, such as Mycobacterium avium complex, cytomegalovirus, and cryptococcus.[93] Sarcoidosis is confused most commonly with neoplastic diseases, such as lymphoma, or with disorders characterized also by a mononuclear cell granulomatous inflammatory process, such as the mycobacterial and fungal disorders.[26]
Chest radiograph changes are divided into four stages:[94]
- bihilar lymphadenopathy
- bihilar lymphadenopathy and reticulonodular infiltrates
- bilateral pulmonary infiltrates
- fibrocystic sarcoidosis typically with upward hilar retraction, cystic and bullous changes
Although people with stage 1 radiographs tend to have the acute or subacute, reversible form of the disease, those with stages 2 and 3 often have the chronic, progressive disease; these patterns do not represent consecutive "stages" of sarcoidosis. Thus, except for epidemiologic purposes, this categorization is mostly of historic interest.[26]
In sarcoidosis presenting in the Caucasian population, hilar adenopathy and erythema nodosum are the most common initial symptoms. In this population, a biopsy of the gastrocnemius muscle is a useful tool in correctly diagnosing the person. The presence of a noncaseating epithelioid granuloma in a gastrocnemius specimen is definitive evidence of sarcoidosis, as other tuberculoid and fungal diseases extremely rarely present histologically in this muscle.[95]
MRI is one modality for diagnosis cardiac sarcoidosis. It is considered inferior to a PET scan as it cannot easily differentiate active disease from scars or old lesions. PET scans appear to have a higher sensitivity and specificity compared to cardiac MRI.[96] For that reason, PET scans are most commonly used to guide both treatment decisions and diagnosis.[43]
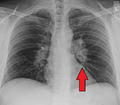 Hilar adenopathy especially on the person's left (AP CXR)
Hilar adenopathy especially on the person's left (AP CXR)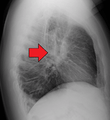 Hilar adenopathy especially on the person's left (lateral CXR)
Hilar adenopathy especially on the person's left (lateral CXR)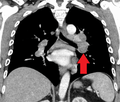 Hilar adenopathy especially on the person's left (coronal CT)
Hilar adenopathy especially on the person's left (coronal CT)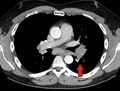 Hilar adenopathy especially on the person's left (transverse CT)
Hilar adenopathy especially on the person's left (transverse CT)
Classification
Sarcoidosis may be divided into the following types:[34]
Treatment
Treatments for sarcoidosis vary greatly depending on the patient.[97] At least half of patients require no systemic therapy.[98] Most persons (>75%) only require symptomatic treatment with non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen or aspirin.[99] For persons presenting with lung symptoms, unless the respiratory impairment is devastating, active pulmonary sarcoidosis is observed usually without therapy for two to three months; if the inflammation does not subside spontaneously, therapy is instituted.[26]
Major categories of drug interventions include glucocorticoids, antimetabolites, biologic agents especially monoclonal anti-tumor necrosis factor antibodies.[98] Investigational treatments include specific antibiotic combinations and mesenchymal stem cells.[98] If drug intervention is indicated, a step-wise approach is often used to explore alternatives in order of increasing side effects and to monitor potentially toxic effects.[98]
Corticosteroids, most commonly prednisone or prednisolone, have been the standard treatment for many years.[18] In some people, this treatment can slow or reverse the course of the disease, but other people do not respond to steroid therapy. The use of corticosteroids in mild disease is controversial because in many cases the disease remits spontaneously.[100][101]
Antimetabolites
Antimetabolites, also categorized as steroid-sparing agents, such as azathioprine, methotrexate, mycophenolic acid, and leflunomide[102][103] are often used as alternatives to corticosteroids.[18][104] Of these, methotrexate is most widely used and studied.[104][105] Methotrexate is considered a first-line treatment in neurosarcoidosis, often in conjunction with corticosteroids.[49][104] Long-term treatment with methotrexate is associated with liver damage in about 10% of people and hence may be a significant concern in people with liver involvement and requires regular liver function test monitoring.[18] Methotrexate can also lead to pulmonary toxicity (lung damage), although this is fairly uncommon and more commonly it can confound the leukopenia caused by sarcoidosis.[18] Due to these safety concerns it is often recommended that methotrexate is combined with folic acid in order to prevent toxicity.[18] Azathioprine treatment can also lead to liver damage.[105] Leflunomide is being used as a replacement for methotrexate, possibly due to its purportedly lower rate of pulmonary toxicity.[105] Mycophenolic acid has been used successfully in uveal sarcoidosis,[106] neurosarcoidosis (especially CNS sarcoidosis; minimally effective in sarcoidosis myopathy),[107] and pulmonary sarcoidosis.[108][109]
Immunosuppressants
As the granulomas are caused by collections of immune system cells, particularly T cells, there has been some success using immunosuppressants (like cyclophosphamide, cladribine,[110] chlorambucil, and cyclosporine), immunomodulatory (pentoxifylline and thalidomide), and anti-tumor necrosis factor treatment[111][112] (such as infliximab, etanercept, golimumab, and adalimumab).[99][113][114]
In a clinical trial cyclosporine added to prednisone treatment failed to demonstrate any significant benefit over prednisone alone in people with pulmonary sarcoidosis, although there was evidence of increased toxicity from the addition of cyclosporine to the steroid treatment including infections, malignancies (cancers), hypertension, and kidney dysfunction.[105] Likewise chlorambucil and cyclophosphamide are seldom used in the treatment of sarcoidosis due to their high degree of toxicity, especially their potential for causing malignancies.[115] Infliximab has been used successfully to treat pulmonary sarcoidosis in clinical trials in a number of persons.[105] Etanercept, on the other hand, has failed to demonstrate any significant efficacy in people with uveal sarcoidosis in a couple of clinical trials.[105] Likewise golimumab has failed to show any benefit in persons with pulmonary sarcoidosis.[105] One clinical trial of adalimumab found treatment response in about half of subjects, which is similar to that seen with infliximab, but as adalimumab has better tolerability profile it may be preferred over infliximab.[105]
Specific organ treatments
Ursodeoxycholic acid has been used successfully as a treatment for cases with liver involvement.[116] Thalidomide has also been tried successfully as a treatment for treatment-resistant lupus pernio in a clinical trial, which may stem from its anti-TNF activity, although it failed to exhibit any efficacy in a pulmonary sarcoidosis clinical trial.[86][113] Cutaneous disease may be successfully managed with antimalarials (such as chloroquine and hydroxychloroquine) and the tetracycline antibiotic, minocycline.[26][113] Antimalarials have also demonstrated efficacy in treating sarcoidosis-induced hypercalcemia and neurosarcoidosis.[18] Long-term use of antimalarials is limited, however, by their potential to cause irreversible blindness and hence the need for regular ophthalmologic screening.[115] This toxicity is usually less of a problem with hydroxychloroquine than with chloroquine, although hydroxychloroquine can disturb the glucose homeostasis.[115]
Recently selective phosphodiesterase 4 (PDE4) inhibitors like apremilast (a thalidomide derivative), roflumilast, and the less subtype-selective PDE4 inhibitor, pentoxifylline, have been tried as a treatment for sarcoidosis, with successful results being obtained with apremilast in cutaneous sarcoidosis in a small open-label study.[117][118] Pentoxifylline has been used successfully to treat acute disease although its use is greatly limited by its gastrointestinal toxicity (mostly nausea, vomiting, and diarrhea).[103][105][115] Case reports have supported the efficacy of rituximab, an anti-CD20 monoclonal antibody and a clinical trial investigating atorvastatin as a treatment for sarcoidosis is under-way.[119][120] ACE inhibitors have been reported to cause remission in cutaneous sarcoidosis and improvement in pulmonary sarcoidosis, including improvement in pulmonary function, remodeling of lung parenchyma and prevention of pulmonary fibrosis in separate case series'.[121][122][123] Nicotine patches have been found to possess anti-inflammatory effects in sarcoidosis patients, although whether they had disease-modifying effects requires further investigation.[124] Antimycobacterial treatment (drugs that kill off mycobacteria, the causative agents behind tuberculosis and leprosy) has also proven itself effective in treating chronic cutaneous (that is, it affects the skin) sarcoidosis in one clinical trial.[125] Quercetin has also been tried as a treatment for pulmonary sarcoidosis with some early success in one small trial.[126]
Because of its uncommon nature, the treatment of male reproductive tract sarcoidosis is controversial. Since the differential diagnosis includes testicular cancer, some recommend orchiectomy, even if evidence of sarcoidosis in other organs is present. In the newer approach, testicular, epididymal biopsy and resection of the largest lesion has been proposed.[62]
Symptoms
People with sarcoidosis may have a range of symptoms that do not correspond with objective physical evidence of disease but that still decrease quality of life.[127]
Physical therapy, rehabilitation, and counseling can help avoid deconditioning,[127]:733 and improve social participation, psychological well-being, and activity levels. Key aspects are avoiding exercise intolerance and muscle weakness.[127]:734
Low or moderate-intensity physical training has been shown to improve fatigue, psychological health, and physical functioning in people sarcoidosis without adverse effects.[128][129] Inspiratory muscle training has also decreased severe fatigue perception in subjects with early stages of sarcoidosis, as well as improving functional and maximal exercise capacity and respiratory muscle strength.[130] The duration, frequency, and physical intensity of exercise needs to accommodate impairments such as joint pain, muscle pain, and fatigue.[127]:734[129][131]
Neurostimulants such as methylphenidate and modafinil have shown some effectiveness as an adjunct for treatment of sarcoidosis fatigue.[127]:733[132]
Treatments for symptomatic neuropathic pain in sarcoidosis patients is similar to that for other causes, and include antidepressants, anticonvulsants and prolonged-release opioids, however, only 30 to 60% of patients experience limited pain relief.[127]:733
Prognosis
The disease can remit spontaneously or become chronic, with exacerbations and remissions. In some persons, it can progress to pulmonary fibrosis and death. In benign cases, remission can occur in 24 to 36 months without treatment but regular follow ups are required. Some cases, however, may persist several decades.[18] Two-thirds of people with the condition achieve a remission within 10 years of the diagnosis.[133] When the heart is involved, the prognosis is generally less favourable, though corticosteroids appear effective in improving AV conduction.[134][135] The prognosis tends to be less favourable in African Americans than in white Americans.[24] In a Swedish population-based analysis, the majority of cases who did not have severe disease at diagnosis had comparable mortality to the general population.[136] The risk for premature death was markedly (2.3-fold) increased compared to the general population for a smaller group of cases with severe disease at diagnosis.[136]
Some 1990s studies indicated that persons with sarcoidosis appear to be at significantly increased risk for cancer, in particular lung cancer, lymphomas,[137] and cancer in other organs known to be affected in sarcoidosis.[138][139] In sarcoidosis-lymphoma syndrome, sarcoidosis is followed by the development of a lymphoproliferative disorder such as non-Hodgkin lymphoma.[140] This may be attributed to the underlying immunological abnormalities that occur during the sarcoidosis disease process.[141] Sarcoidosis can also follow cancer[142][143] or occur concurrently with cancer.[144][145] There have been reports of hairy cell leukemia,[146] acute myeloid leukemia,[147] and acute myeloblastic leukemia[148] associated with sarcoidosis. Sometimes, sarcoidosis, even untreated, can be complicated by opportunistic infections.[149][150]
Epidemiology
Sarcoidosis most commonly affects young adults of both sexes, although studies have reported more cases in females. Incidence is highest for individuals younger than 40 and peaks in the age-group from 20 to 29 years; a second peak is observed for women over 50.[18][134]
Sarcoidosis occurs throughout the world in all races with an average incidence of 16.5 per 100,000 in men and 19 per 100,000 in women. The disease is most common in Northern European countries and the highest annual incidence of 60 per 100,000 is found in Sweden and Iceland. In the United Kingdom the prevalence is 16 in 100,000.[151] In the United States, sarcoidosis is more common in people of African descent than Caucasians, with annual incidence reported as 35.5 and 10.9 per 100,000, respectively.[152] Sarcoidosis is less commonly reported in South America, Spain, India, Canada, and the Philippines. There may be a higher susceptibility to sarcoidosis in those with celiac disease. An association between the two disorders has been suggested.[153]
There also has been a seasonal clustering observed in sarcoidosis-affected individuals.[154] In Greece about 70% of diagnoses occur between March and May every year, in Spain about 50% of diagnoses occur between April and June, and in Japan it is mostly diagnosed during June and July.[154]
The differing incidence across the world may be at least partially attributable to the lack of screening programs in certain regions of the world, and the overshadowing presence of other granulomatous diseases, such as tuberculosis, that may interfere with the diagnosis of sarcoidosis where they are prevalent.[134] There may also be differences in the severity of the disease between people of different ethnicities. Several studies suggest the presentation in people of African origin may be more severe and disseminated than for Caucasians, who are more likely to have asymptomatic disease.[64] Manifestation appears to be slightly different according to race and sex. Erythema nodosum is far more common in men than in women and in Caucasians than in other races. In Japanese persons, ophthalmologic and cardiac involvement are more common than in other races.[18]
It is more common in certain occupations, namely firefighters, educators, military personnel, persons who work in industries where pesticides are used, law enforcement, and healthcare personnel.[155] In the year after the September 11 attacks, the rate of sarcoidosis incidence went up four-fold (to 86 cases per 100,000).[29][155]
History
It was first described in 1877 by Dr. Jonathan Hutchinson, a dermatologist as a condition causing red, raised rashes on the face, arms, and hands.[15] In 1889 the term Lupus pernio was coined by Dr. Ernest Besnier, another dermatologist.[156] Later in 1892 lupus pernio's histology was defined.[156] In 1902 bone involvement was first described by a group of three doctors.[156] Between 1909 and 1910 uveitis in sarcoidosis was first described, and later in 1915 it was emphasised, by Dr. Schaumann, that it was a systemic condition.[156] This same year lung involvement was also described.[156] In 1937 uveoparotid fever was first described and likewise in 1941 Löfgren syndrome was first described.[156] In 1958 the first international conference on sarcoidosis was called in London, likewise the first USA sarcoidosis conference occurred in Washington, DC in the year 1961.[156] It has also been called Besnier-Boeck disease or Besnier-Boeck-Schaumann disease.[157]
Etymology
The word "sarcoidosis" comes from Greek [σάρκο-] sarcο- meaning "flesh", the suffix -(e)ido (from the Greek εἶδος -eidos [usually omitting the initial e in English as the diphthong epsilon-iota in Classic Greek stands for a long "i" = English ee]) meaning "type", " resembles" or "like", and -sis, a common suffix in Greek meaning "condition". Thus the whole word means "a condition that resembles crude flesh". The first cases of sarcoïdosis, which were recognised as a new pathological entity, in Scandinavia, at the end of the 19th century exhibited skin nodules resembling cutaneous sarcomas, hence the name initially given.
Society and culture
The World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) is an organisation of physicians involved in the diagnosis and treatment of sarcoidosis and related conditions.[158] WASOG publishes the journal Sarcoidosis, Vasculitis and Diffuse Lung Diseases.[159] Additionally, the Foundation for Sarcoidosis Research (FSR) is devoted to supporting research into sarcoidosis and its possible treatments.[160]
There have been concerns that World Trade Center rescue workers are at a heightened risk for sarcoidosis.[161][162]
Comedian and actor Bernie Mac had sarcoidosis. In 2005, he mentioned that the disease was in remission.[163] His death on 9 August 2008 was caused by complications from pneumonia, though Mac's agent states the sarcoidis was not related to his fatal pneumonia.[164]
Karen "Duff" Duffy, MTV personality and actress, was diagnosed with neurosarcoidosis in 1995.[165]
American football player Reggie White died in 2004, with pulmonary and cardiac sarcoidosis being contributing factors to his fatal heart arrhythmia.[166]
Singer Sean Levert died in 2008 of sarcoidosis complications.[167]
Joseph Rago, Pulitzer Prize winning writer known for his work at The Wall Street Journal, died of sarcoidosis complications in 2017.[168]
Several historical figures are suspected of having suffered from sarcoidosis. In a 2014 letter to the British medical journal The Lancet, it was suggested that the French Revolution leader Maximilien Robespierre may have had sarcoidosis, causing him impairment during his time as head of the Reign of Terror.[169] The symptoms associated with Ludwig van Beethoven's 1827 death have been described as possibly consistent with sarcoidosis.[170] Author Robert Louis Stevenson (1850-1894) had a history of chronic coughs and chest complaints, and sarcoidosis has been suggested as a diagnosis.[171]
Pregnancy
Sarcoidosis generally does not prevent successful pregnancy and delivery; the increase in estrogen levels during pregnancy may even have a slightly beneficial immunomodulatory effect. In most cases, the course of the disease is unaffected by pregnancy, with improvement in a few cases and worsening of symptoms in very few cases, although it is worth noting that a number of the immunosuppressants (such as methotrexate, cyclophosphamide) used in corticosteroid-refractory sarcoidosis are known teratogens.[172]
References
- Konstantinidis, G. (2005). Elsevier's Dictionary of Medicine and Biology: in English, Greek, German, Italian and Latin. Elsevier. p. 1454. ISBN 9780080460123.
- "What Is Sarcoidosis?". NHLBI. June 14, 2013. Archived from the original on 6 April 2016. Retrieved 28 March 2016.
- "What Are the Signs and Symptoms of Sarcoidosis?". NHLBI. June 14, 2013. Archived from the original on 7 April 2016. Retrieved 29 March 2016.
- "Who Is at Risk for Sarcoidosis?". NHLBI. June 14, 2013. Archived from the original on 7 April 2016. Retrieved 28 March 2016.
- Wijsenbeek MS, Culver DA (December 2015). "Treatment of Sarcoidosis". Clinics in Chest Medicine. 36 (4): 751–67. doi:10.1016/j.ccm.2015.08.015. PMID 26593147.
- Govender P, Berman JS (December 2015). "The Diagnosis of Sarcoidosis". Clinics in Chest Medicine. 36 (4): 585–602. doi:10.1016/j.ccm.2015.08.003. PMID 26593135.
- Ferri, Fred F. (2010). Ferri's differential diagnosis : a practical guide to the differential diagnosis of symptoms, signs, and clinical disorders (2nd ed.). Philadelphia, PA: Elsevier/Mosby. p. Chapter S. ISBN 978-0323076999.
- Drent M, Cremers JP, Jansen TL (May 2014). "Pulmonology meets rheumatology in sarcoidosis: a review on the therapeutic approach". Current Opinion in Rheumatology. 26 (3): 276–84. doi:10.1097/bor.0000000000000052. PMID 24614277.
- Judson MA (February 2016). "Corticosteroids in Sarcoidosis". Rheumatic Diseases Clinics of North America. 42 (1): 119–35, ix. doi:10.1016/j.rdc.2015.08.012. PMID 26611555.
- GBD 2015 Disease Injury Incidence Prevalence Collaborators (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- GBD 2015 Mortality Causes of Death Collaborators (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- Baughman RP, Culver DA, Judson MA (March 2011). "A concise review of pulmonary sarcoidosis". American Journal of Respiratory and Critical Care Medicine. 183 (5): 573–81. doi:10.1164/rccm.201006-0865CI. PMC 3081278. PMID 21037016.
- "What Causes Sarcoidosis?". NHLBI. June 14, 2013. Archived from the original on 6 April 2016. Retrieved 28 March 2016.
- Kobak S (October 2015). "Sarcoidosis: a rheumatologist's perspective". Therapeutic Advances in Musculoskeletal Disease. 7 (5): 196–205. doi:10.1177/1759720x15591310. PMC 4572362. PMID 26425148.
- James DG, Sharma OP (September 2002). "From Hutchinson to now: a historical glimpse" (PDF). Current Opinion in Pulmonary Medicine. 8 (5): 416–23. doi:10.1097/00063198-200209000-00013. PMID 12172446. Archived (PDF) from the original on 2016-03-04.
- "Lung Diseases: Sarcoidosis: Signs & Symptoms". National Heart, Lung, and Blood Institute. Archived from the original on May 7, 2009. Retrieved May 9, 2009.
- Kamangar, N; Rohani, P; Shorr, AF (6 February 2014). Peters, SP; Talavera, F; Rice, TD; Mosenifar, Z (eds.). "Sarcoidosis Clinical Presentation". Medscape Reference. WebMD. Archived from the original on 25 February 2014. Retrieved 19 February 2014.
- Nunes H, Bouvry D, Soler P, Valeyre D (November 2007). "Sarcoidosis". Orphanet Journal of Rare Diseases. 2: 46. doi:10.1186/1750-1172-2-46. PMC 2169207. PMID 18021432.
- King, TE Jr. (March 2008). "Sarcoidosis: Interstitial Lung Diseases: Merck Manual Home Edition". The Merck Manual Home Edition. Merck Sharp & Dohme Corp. Archived from the original on 20 February 2014. Retrieved 19 February 2014.
- Sweiss NJ, Patterson K, Sawaqed R, Jabbar U, Korsten P, Hogarth K, Wollman R, Garcia JG, Niewold TB, Baughman RP (August 2010). "Rheumatologic manifestations of sarcoidosis". Seminars in Respiratory and Critical Care Medicine. 31 (4): 463–73. doi:10.1055/s-0030-1262214. PMC 3314339. PMID 20665396.
- Holmes J, Lazarus A (November 2009). "Sarcoidosis: extrathoracic manifestations". Disease-A-Month. 55 (11): 675–92. doi:10.1016/j.disamonth.2009.05.002. PMID 19857642.
- Dempsey OJ, Paterson EW, Kerr KM, Denison AR (August 2009). "Sarcoidosis". BMJ. 339: b3206. doi:10.1136/bmj.b3206. PMID 19717499.
- Tolaney SM, Colson YL, Gill RR, Schulte S, Duggan MM, Shulman LN, Winer EP (October 2007). "Sarcoidosis mimicking metastatic breast cancer". Clinical Breast Cancer. 7 (10): 804–10. doi:10.3816/CBC.2007.n.044. PMID 18021484.
- Kamangar, N; Rohani, P; Shorr, AF (6 February 2014). Peters, SP; Talavera, F; Rice, TD; Mosenifar, Z (eds.). "Sarcoidosis". Medscape Reference. WebMD. Archived from the original on 10 February 2014. Retrieved 19 February 2014.
- Baughman RP, Lower EE, Gibson K (June 2012). "Pulmonary manifestations of sarcoidosis". Presse Médicale. 41 (6 Pt 2): e289–302. doi:10.1016/j.lpm.2012.03.019. PMID 22579234.
- Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J (2011). Harrison's Principles of Internal Medicine (18th ed.). New York: McGraw-Hill Professional. ISBN 978-0-07174889-6.
- Fuhrer G, Myers JN (November 2009). "Intrathoracic sarcoidosis". Disease-A-Month. 55 (11): 661–74. doi:10.1016/j.disamonth.2009.04.009. PMID 19857641.
- Nunes H, Uzunhan Y, Freynet O, Humbert M, Brillet PY, Kambouchner M, Valeyre D (June 2012). "Pulmonary hypertension complicating sarcoidosis". Presse Médicale. 41 (6 Pt 2): e303–16. doi:10.1016/j.lpm.2012.04.003. PMID 22608948.
- Chen ES, Moller DR (July 2011). "Sarcoidosis--scientific progress and clinical challenges". Nature Reviews. Rheumatology. 7 (8): 457–67. doi:10.1038/nrrheum.2011.93. PMID 21750528.
- Kumar and Clark, Clinical Medicine, 8th edition, p. 846.
- Mañá J, Marcoval J (June 2012). "Skin manifestations of sarcoidosis". Presse Médicale. 41 (6 Pt 2): e355–74. doi:10.1016/j.lpm.2012.02.046. PMID 22579238.
- Heath CR, David J, Taylor SC (January 2012). "Sarcoidosis: Are there differences in your skin of color patients?". Journal of the American Academy of Dermatology. 66 (1): 121.e1–14. doi:10.1016/j.jaad.2010.06.068. PMID 22000704.
- Lodha S, Sanchez M, Prystowsky S (August 2009). "Sarcoidosis of the skin: a review for the pulmonologist". Chest. 136 (2): 583–596. doi:10.1378/chest.08-1527. PMID 19666758.
- James, WD; Berger, T; Dirk, M (2006). Andrew's Diseases of the Skin: Clinical Dermatology (10th ed.). Philadelphia: Saunders Elsevier. pp. 708–711. ISBN 978-0808923510.
- House NS, Welsh JP, English JC (August 2012). "Sarcoidosis-induced alopecia". Dermatology Online Journal. 18 (8): 4. PMID 22948054. Archived from the original on 2 March 2014.
- Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K (July 2014). "HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis". Heart Rhythm. 11 (7): 1305–23. doi:10.1016/j.hrthm.2014.03.043. PMID 24819193.
- Doughan AR, Williams BR (February 2006). "Cardiac sarcoidosis". Heart. 92 (2): 282–8. doi:10.1136/hrt.2005.080481. PMC 1860791. PMID 16415205.
- Youssef G, Beanlands RS, Birnie DH, Nery PB (December 2011). "Cardiac sarcoidosis: applications of imaging in diagnosis and directing treatment". Heart. 97 (24): 2078–87. doi:10.1136/hrt.2011.226076. PMID 22116891.
- Reuhl J, Schneider M, Sievert H, Lutz FU, Zieger G (October 1997). "Myocardial sarcoidosis as a rare cause of sudden cardiac death". Forensic Science International. 89 (3): 145–53. doi:10.1016/S0379-0738(97)00106-0. PMID 9363623.
- Rajasenan V, Cooper ES (July 1969). "Myocardial sarcoidosis, bouts of ventricular tachycardia, psychiatric manifestations and sudden death. A case report". Journal of the National Medical Association. 61 (4): 306–9. PMC 2611747. PMID 5796402.
- Chapelon-Abric C (June 2012). "Cardiac sarcoidosis". Presse Médicale. 41 (6 Pt 2): e317–30. doi:10.1016/j.lpm.2012.04.002. PMID 22608949.
- Hulten E, Aslam S, Osborne M, Abbasi S, Bittencourt MS, Blankstein R (February 2016). "Cardiac sarcoidosis-state of the art review". Cardiovascular Diagnosis and Therapy. 6 (1): 50–63. doi:10.3978/j.issn.2223-3652.2015.12.13. PMC 4731586. PMID 26885492.
- "About Sarcoidosis". Stanford University Sarcoidosis Program. Retrieved 2019-08-09.
- Sekhri, Vishal; Sanal, Shireen; DeLorenzo, Lawrence J.; Aronow, Wilbert S.; Maguire, George P. (2011-08-01). "Cardiac sarcoidosis: a comprehensive review". Archives of Medical Science. 7 (4): 546–554. doi:10.5114/aoms.2011.24118. ISSN 1734-1922. PMC 3258766. PMID 22291785.
- Bodaghi B, Touitou V, Fardeau C, Chapelon C, LeHoang P (June 2012). "Ocular sarcoidosis". Presse Médicale. 41 (6 Pt 2): e349–54. doi:10.1016/j.lpm.2012.04.004. PMID 22595776.
- Jamilloux Y, Kodjikian L, Broussolle C, Sève P (August 2014). "Sarcoidosis and uveitis". Autoimmunity Reviews. 13 (8): 840–9. doi:10.1016/j.autrev.2014.04.001. PMID 24704868.
- Papadia M, Herbort CP, Mochizuki M (December 2010). "Diagnosis of ocular sarcoidosis". Ocular Immunology and Inflammation. 18 (6): 432–41. doi:10.3109/09273948.2010.524344. PMID 21091056.
- Qazi FA, Thorne JE, Jabs DA (October 2003). "Scleral nodule associated with sarcoidosis". American Journal of Ophthalmology. 136 (4): 752–4. doi:10.1016/S0002-9394(03)00454-9. PMID 14516826.
- Nozaki K, Judson MA (June 2012). "Neurosarcoidosis: Clinical manifestations, diagnosis and treatment". Presse Médicale. 41 (6 Pt 2): e331–48. doi:10.1016/j.lpm.2011.12.017. PMID 22595777.
- Said G, Lacroix C, Planté-Bordeneuve V, Le Page L, Pico F, Presles O, Senant J, Remy P, Rondepierre P, Mallecourt J (February 2002). "Nerve granulomas and vasculitis in sarcoid peripheral neuropathy: a clinicopathological study of 11 patients". Brain. 125 (Pt 2): 264–75. doi:10.1093/brain/awf027. PMID 11844727.
- Vardhanabhuti V, Venkatanarasimha N, Bhatnagar G, Maviki M, Iyengar S, Adams WM, Suresh P (March 2012). "Extra-pulmonary manifestations of sarcoidosis". Clinical Radiology. 67 (3): 263–76. doi:10.1016/j.crad.2011.04.018. PMID 22094184.
- Tavee J, Culver D (June 2011). "Sarcoidosis and small-fiber neuropathy". Current Pain and Headache Reports. 15 (3): 201–6. doi:10.1007/s11916-011-0180-8. PMID 21298560.
- Heij L, Dahan A, Hoitsma E (2012). "Sarcoidosis and pain caused by small-fiber neuropathy". Pain Research and Treatment. 2012: 1–6. doi:10.1155/2012/256024. PMC 3523152. PMID 23304492.
- Porter N, Beynon HL, Randeva HS (August 2003). "Endocrine and reproductive manifestations of sarcoidosis". QJM. 96 (8): 553–61. doi:10.1093/qjmed/hcg103. PMID 12897340.
- Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL (August 1981). "Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D". The New England Journal of Medicine. 305 (8): 440–3. doi:10.1056/NEJM198108203050807. PMID 6894783.
- Rheumatology Diagnosis & Therapies (2nd ed.). Philadelphia: Lippincott Williams & Wilkins. 2005. p. 342.
- Antonelli A, Fazzi P, Fallahi P, Ferrari SM, Ferrannini E (August 2006). "Prevalence of hypothyroidism and Graves disease in sarcoidosis". Chest. 130 (2): 526–32. doi:10.1378/chest.130.2.526. PMID 16899854.
- Manchanda A, Patel S, Jiang JJ, Babu AR (March–April 2013). "Thyroid: an unusual hideout for sarcoidosis" (PDF). Endocrine Practice. 19 (2): e40–3. doi:10.4158/EP12131.CR. PMID 23337134.
- Fausto N, Abbas A (2004). Robbins and Cotran Pathologic Basis of disease (7th ed.). Philadelphia, PA: Elsevier/Saunders. pp. 737–9. ISBN 978-0721601878.
- Tokala H, Polsani K, Kalavakunta JK (2013-01-01). "Gastric sarcoidosis: a rare clinical presentation". Case Reports in Gastrointestinal Medicine. 2013: 260704. doi:10.1155/2013/260704. PMC 3867894. PMID 24368949.
- Vera C, Funaro D, Bouffard D (July–August 2013). "Vulvar sarcoidosis: case report and review of the literature". Journal of Cutaneous Medicine and Surgery. 17 (4): 287–90. doi:10.2310/7750.2012.12083. PMID 23815963.
- Paknejad O, Gilani MA, Khoshchehreh M (April 2011). "Testicular masses in a man with a plausible sarcoidosis". Indian Journal of Urology. 27 (2): 269–71. doi:10.4103/0970-1591.82848. PMC 3142840. PMID 21814320.
- Cremers JP, Drent M, Baughman RP, Wijnen PA, Koek GH (September 2012). "Therapeutic approach of hepatic sarcoidosis". Current Opinion in Pulmonary Medicine. 18 (5): 472–82. doi:10.1097/MCP.0b013e3283541626. PMID 22617809.
- "Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999". American Journal of Respiratory and Critical Care Medicine. 160 (2): 736–55. August 1999. doi:10.1164/ajrccm.160.2.ats4-99. PMID 10430755.
- Wurm K, Löhr G (March 1986). "Immuno-cytological blood tests in cases of sarcoidosis". Sarcoidosis. 3 (1): 52–9. PMID 3033787.
- Chen HC, Kang BH, Lai CT, Lin YS (July 2005). "Sarcoidal granuloma in cervical lymph nodes". Journal of the Chinese Medical Association. 68 (7): 339–42. doi:10.1016/S1726-4901(09)70172-8. PMID 16038376.
- Rao DA, Dellaripa PF (May 2013). "Extrapulmonary manifestations of sarcoidosis". Rheumatic Diseases Clinics of North America. 39 (2): 277–97. doi:10.1016/j.rdc.2013.02.007. PMC 3756667. PMID 23597964.
- Shimamura Y, Taniguchi Y, Yoshimatsu R, Kawase S, Yamagami T, Terada Y (January 2016). "Granulomatous periostitis and tracheal involvement in sarcoidosis". Rheumatology. 55 (1): 102. doi:10.1093/rheumatology/kev319. PMID 26320137.
- Korkmaz C, Efe B, Tel N, Kabukçuoglu S, Erenoglu E (March 1999). "Sarcoidosis with palpable nodular myositis, periostitis and large-vessel vasculitis stimulating Takayasu's arteritis". Rheumatology. 38 (3): 287–8. doi:10.1093/rheumatology/38.3.287. PMID 10325674.
- Rossman MD, Kreider ME (August 2007). "Lesson learned from ACCESS (A Case Controlled Etiologic Study of Sarcoidosis)". Proceedings of the American Thoracic Society. 4 (5): 453–6. doi:10.1513/pats.200607-138MS. PMID 17684288.
- Cathcart S, Sami N, Elewski B (May 2012). "Sarcoidosis as an adverse effect of tumor necrosis factor inhibitors". Journal of Drugs in Dermatology. 11 (5): 609–12. PMID 22527429.
- Rossides, Marios; Grunewald, Johan; Eklund, Anders; Kullberg, Susanna; Giuseppe, Daniela Di; Askling, Johan; Arkema, Elizabeth V. (2018-01-01). "Familial Aggregation and Heritability of Sarcoidosis: A Swedish Nested Case-Control Study". European Respiratory Journal. 52 (2): 1800385. doi:10.1183/13993003.00385-2018. ISSN 0903-1936. PMID 29946010.
- Iannuzzi MC (August 2007). "Advances in the genetics of sarcoidosis". Proceedings of the American Thoracic Society. 4 (5): 457–60. doi:10.1513/pats.200606-136MS. PMID 17684289.
- Spagnolo P, Grunewald J (May 2013). "Recent advances in the genetics of sarcoidosis". Journal of Medical Genetics. 50 (5): 290–7. doi:10.1136/jmedgenet-2013-101532. PMID 23526832.
- Grunewald J, Eklund A, Olerup O (March 2004). "Human leukocyte antigen class I alleles and the disease course in sarcoidosis patients". American Journal of Respiratory and Critical Care Medicine. 169 (6): 696–702. CiteSeerX 10.1.1.321.2788. doi:10.1164/rccm.200303-459OC. PMID 14656748.
- Grunewald J (August 2010). "Review: role of genetics in susceptibility and outcome of sarcoidosis". Seminars in Respiratory and Critical Care Medicine. 31 (4): 380–9. doi:10.1055/s-0030-1262206. PMID 20665388.
- Gialafos E, Triposkiadis F, Kouranos V, Rapti A, Kosmas I, Manali E, Giamouzis G, Elezoglou A, Peros I, Anagnostopoulou O, Koulouris N, Gazouli M (2014-12-01). "Relationship between tumor necrosis factor-α (TNFA) gene polymorphisms and cardiac sarcoidosis". In Vivo. 28 (6): 1125–9. PMID 25398810.
- Saidha S, Sotirchos ES, Eckstein C (March 2012). "Etiology of sarcoidosis: does infection play a role?". The Yale Journal of Biology and Medicine. 85 (1): 133–41. PMC 3313528. PMID 22461752.
- Müller-Quernheim J, Prasse A, Zissel G (June 2012). "Pathogenesis of sarcoidosis". Presse Médicale. 41 (6 Pt 2): e275–87. doi:10.1016/j.lpm.2012.03.018. PMID 22595775.
- Gupta D, Agarwal R, Aggarwal AN, Jindal SK (September 2007). "Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis". The European Respiratory Journal. 30 (3): 508–16. doi:10.1183/09031936.00002607. PMID 17537780.
- Almenoff PL, Johnson A, Lesser M, Mattman LH (May 1996). "Growth of acid fast L forms from the blood of patients with sarcoidosis". Thorax. 51 (5): 530–3. doi:10.1136/thx.51.5.530. PMC 473601. PMID 8711683.
- Morgenthau AS, Iannuzzi MC (January 2011). "Recent advances in sarcoidosis" (PDF). Chest. 139 (1): 174–82. doi:10.1378/chest.10-0188. PMID 21208877.
- Padilla ML, Schilero GJ, Teirstein AS (March 2002). "Donor-acquired sarcoidosis". Sarcoidosis, Vasculitis, and Diffuse Lung Diseases. 19 (1): 18–24. PMID 12002380.
- Romagnani S (June 1997). "The Th1/Th2 paradigm". Immunology Today. 18 (6): 263–6. doi:10.1016/S0167-5699(97)80019-9. PMID 9190109.
- Morell F, Levy G, Orriols R, Ferrer J, De Gracia J, Sampol G (April 2002). "Delayed cutaneous hypersensitivity tests and lymphopenia as activity markers in sarcoidosis". Chest. 121 (4): 1239–44. doi:10.1378/chest.121.4.1239. PMID 11948059.
- Bargagli E, Olivieri C, Rottoli P (December 2011). "Cytokine modulators in the treatment of sarcoidosis". Rheumatology International. 31 (12): 1539–44. doi:10.1007/s00296-011-1969-9. hdl:11365/35698. PMID 21644041.
- Kettritz R, Goebel U, Fiebeler A, Schneider W, Luft F (October 2006). "The protean face of sarcoidosis revisited" (PDF). Nephrology, Dialysis, Transplantation. 21 (10): 2690–4. doi:10.1093/ndt/gfl369. PMID 16861724.
- Verschueren K, Van Essche E, Verschueren P, Taelman V, Westhovens R (November 2007). "Development of sarcoidosis in etanercept-treated rheumatoid arthritis patients". Clinical Rheumatology. 26 (11): 1969–71. doi:10.1007/s10067-007-0594-1. PMID 17340045.
- Parrish S, Turner JF (November 2009). "Diagnosis of sarcoidosis". Disease-A-Month. 55 (11): 693–703. doi:10.1016/j.disamonth.2009.06.001. PMID 19857643.
- Hawtin KE, Roddie ME, Mauri FA, Copley SJ (August 2010). "Pulmonary sarcoidosis: the 'Great Pretender'". Clinical Radiology. 65 (8): 642–50. doi:10.1016/j.crad.2010.03.004. PMID 20599067.
- Miliauskas S, Zemaitis M, Sakalauskas R (2010). "Sarcoidosis--moving to the new standard of diagnosis?" (PDF). Medicina. 46 (7): 443–6. doi:10.3390/medicina46070063. PMID 20966615. Archived (PDF) from the original on 2014-02-26.
- Kamangar, N; Rohani, P; Shorr, AF (6 February 2014). Peters, SP; Talavera, F; Rice, TD; Mosenifar, Z (eds.). "Sarcoidosis Workup". Medscape Reference. WebMD. Archived from the original on 1 March 2014. Retrieved 19 February 2014.
- Allmendinger A, Perone R (2009). "Case of the Month". Diagnostic Imaging. 31 (9): 10.
- Joanne Mambretti (2004). "Chest X-ray Stages of Sarcoidosis" (PDF). Journal of Insurance Medicine: 91–92. Archived (PDF) from the original on July 9, 2014. Retrieved June 3, 2012.
- Andonopoulos AP, Papadimitriou C, Melachrinou M, Meimaris N, Vlahanastasi C, Bounas A, Georgiou P (2001). "Asymptomatic gastrocnemius muscle biopsy: an extremely sensitive and specific test in the pathologic confirmation of sarcoidosis presenting with hilar adenopathy" (PDF). Clinical and Experimental Rheumatology. 19 (5): 569–72. PMID 11579718. Archived from the original on 2016-02-24.
- "FDG-PET is a Superior Tool in the Diagnosis and Management of Cardiac Sarcoidosis". American College of Cardiology. Retrieved 2019-08-12.
- Baughman RP, Lower EE (August 2015). "Treatment of Sarcoidosis". Clinical Reviews in Allergy & Immunology. 49 (1): 79–92. doi:10.1007/s12016-015-8492-9. PMID 25989728.
- Baughman RP, Grutters JC (October 2015). "New treatment strategies for pulmonary sarcoidosis: antimetabolites, biological drugs, and other treatment approaches". The Lancet. Respiratory Medicine. 3 (10): 813–22. doi:10.1016/S2213-2600(15)00199-X. PMID 26204816.
- Kamangar, N; Rohani, P; Shorr, AF (6 February 2014). Peters, SP; Talavera, F; Rice, TD; Mosenifar, Z (eds.). "Sarcoidosis Treatment & Management". Medscape Reference. WebMD. Archived from the original on 25 February 2014. Retrieved 19 February 2014.
- White ES, Lynch JP (June 2007). "Current and emerging strategies for the management of sarcoidosis". Expert Opinion on Pharmacotherapy. 8 (9): 1293–311. doi:10.1517/14656566.8.9.1293. PMID 17563264.
- Paramothayan NS, Lasserson TJ, Jones PW (April 2005). "Corticosteroids for pulmonary sarcoidosis". The Cochrane Database of Systematic Reviews (2): CD001114. doi:10.1002/14651858.CD001114.pub2. PMC 6464973. PMID 15846612.
- Sahoo DH, Bandyopadhyay D, Xu M, Pearson K, Parambil JG, Lazar CA, Chapman JT, Culver DA (November 2011). "Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis". The European Respiratory Journal. 38 (5): 1145–50. doi:10.1183/09031936.00195010. PMID 21565914.
- Panselinas E, Judson MA (October 2012). "Acute pulmonary exacerbations of sarcoidosis" (PDF). Chest. 142 (4): 827–836. doi:10.1378/chest.12-1060. PMID 23032450.
- King CS, Kelly W (November 2009). "Treatment of sarcoidosis". Disease-A-Month. 55 (11): 704–18. doi:10.1016/j.disamonth.2009.06.002. PMID 19857644.
- Baughman RP, Nunes H, Sweiss NJ, Lower EE (June 2013). "Established and experimental medical therapy of pulmonary sarcoidosis". The European Respiratory Journal. 41 (6): 1424–38. doi:10.1183/09031936.00060612. PMID 23397302.
- Bhat P, Cervantes-Castañeda RA, Doctor PP, Anzaar F, Foster CS (May–June 2009). "Mycophenolate mofetil therapy for sarcoidosis-associated uveitis". Ocular Immunology and Inflammation. 17 (3): 185–90. doi:10.1080/09273940902862992. PMID 19585361.
- Androdias G, Maillet D, Marignier R, Pinède L, Confavreux C, Broussolle C, Vukusic S, Sève P (March 2011). "Mycophenolate mofetil may be effective in CNS sarcoidosis but not in sarcoid myopathy". Neurology. 76 (13): 1168–72. doi:10.1212/WNL.0b013e318212aafb. PMID 21444902.
- Judson MA (October 2012). "The treatment of pulmonary sarcoidosis". Respiratory Medicine. 106 (10): 1351–61. doi:10.1016/j.rmed.2012.01.013. PMID 22495110.
- Brill AK, Ott SR, Geiser T (2013). "Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study". Respiration; International Review of Thoracic Diseases. 86 (5): 376–83. doi:10.1159/000345596. PMID 23295253.
- Tikoo RK, Kupersmith MJ, Finlay JL (April 2004). "Treatment of refractory neurosarcoidosis with cladribine". The New England Journal of Medicine. 350 (17): 1798–9. doi:10.1056/NEJMc032345. PMID 15103013.
- Maneiro JR, Salgado E, Gomez-Reino JJ, Carmona L (August 2012). "Efficacy and safety of TNF antagonists in sarcoidosis: data from the Spanish registry of biologics BIOBADASER and a systematic review". Seminars in Arthritis and Rheumatism. 42 (1): 89–103. doi:10.1016/j.semarthrit.2011.12.006. PMID 22387045.
- Antoniu SA (January 2010). "Targeting the TNF-alpha pathway in sarcoidosis". Expert Opinion on Therapeutic Targets. 14 (1): 21–9. doi:10.1517/14728220903449244. PMID 20001207.
- Beegle SH, Barba K, Gobunsuy R, Judson MA (2013). "Current and emerging pharmacological treatments for sarcoidosis: a review". Drug Design, Development and Therapy. 7: 325–38. doi:10.2147/DDDT.S31064. PMC 3627473. PMID 23596348.
- Callejas-Rubio JL, López-Pérez L, Ortego-Centeno N (December 2008). "Tumor necrosis factor-alpha inhibitor treatment for sarcoidosis". Therapeutics and Clinical Risk Management. 4 (6): 1305–13. doi:10.2147/TCRM.S967. PMC 2643111. PMID 19337437.
- Dastoori M, Fedele S, Leao JC, Porter SR (April 2013). "Sarcoidosis - a clinically orientated review". Journal of Oral Pathology & Medicine. 42 (4): 281–9. doi:10.1111/j.1600-0714.2012.01198.x. PMID 22845844.
- Bakker GJ, Haan YC, Maillette de Buy Wenniger LJ, Beuers U (October 2012). "Sarcoidosis of the liver: to treat or not to treat?" (PDF). The Netherlands Journal of Medicine. 70 (8): 349–56. PMID 23065982. Archived from the original on 2014-05-18.
- Baughman RP, Judson MA, Ingledue R, Craft NL, Lower EE (February 2012). "Efficacy and safety of apremilast in chronic cutaneous sarcoidosis". Archives of Dermatology. 148 (2): 262–4. doi:10.1001/archdermatol.2011.301. PMID 22004880.
- Clinical trial number NCT01830959 for "Use of Roflumilast to Prevent Exacerbations in Fibrotic Sarcoidosis Patients (REFS)" at ClinicalTrials.gov
- Belkhou A, Younsi R, El Bouchti I, El Hassani S (July 2008). "Rituximab as a treatment alternative in sarcoidosis". Joint, Bone, Spine. 75 (4): 511–2. doi:10.1016/j.jbspin.2008.01.025. PMID 18562234.
- Clinical trial number NCT00279708 for "Atorvastatin to Treat Pulmonary Sarcoidosis" at ClinicalTrials.gov
- Kaura V, Kaura NV, Kaura BN, Kaura CS (2013). "Angiotensin-converting enzyme inhibitors in the treatment of sarcoidosis and association with ACE gene polymorphism: case series". The Indian Journal of Chest Diseases & Allied Sciences. 55 (2): 105–7. PMID 24047001.
- Kaura V, Kaura SH, Kaura CS (2007). "ACE Inhibitor in the treatment of cutaneous and lymphatic sarcoidosis". American Journal of Clinical Dermatology. 8 (3): 183–6. doi:10.2165/00128071-200708030-00006. PMID 17492847.
- Rosenbloom J, Castro SV, Jimenez SA (February 2010). "Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies". Annals of Internal Medicine. 152 (3): 159–66. doi:10.7326/0003-4819-152-3-201002020-00007. PMID 20124232.
- Julian MW, Shao G, Schlesinger LS, Huang Q, Cosmar DG, Bhatt NY, Culver DA, Baughman RP, Wood KL, Crouser ED (February 2013). "Nicotine treatment improves Toll-like receptor 2 and Toll-like receptor 9 responsiveness in active pulmonary sarcoidosis" (PDF). Chest. 143 (2): 461–470. doi:10.1378/chest.12-0383. PMID 22878868.
- Drake WP, Oswald-Richter K, Richmond BW, Isom J, Burke VE, Algood H, Braun N, Taylor T, Pandit KV, Aboud C, Yu C, Kaminski N, Boyd AS, King LE (September 2013). "Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study". JAMA Dermatology. 149 (9): 1040–9. doi:10.1001/jamadermatol.2013.4646. PMC 3927541. PMID 23863960.
- Boots AW, Drent M, de Boer VC, Bast A, Haenen GR (August 2011). "Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis". Clinical Nutrition. 30 (4): 506–12. doi:10.1016/j.clnu.2011.01.010. PMID 21324570.
- Drent M, Strookappe B, Hoitsma E, De Vries J (December 2015). "Consequences of Sarcoidosis". Clinics in Chest Medicine. 36 (4): 727–37. doi:10.1016/j.ccm.2015.08.013. PMID 26593145.
- Marcellis R, Van der Veeke M, Mesters I, Drent M, De Bie R, De Vries G, Lenssen A (June 2015). "Does physical training reduce fatigue in sarcoidosis?". Sarcoidosis, Vasculitis, and Diffuse Lung Diseases. 32 (1): 53–62. PMID 26237356.
- Strookappe B, Swigris J, De Vries J, Elfferich M, Knevel T, Drent M (October 2015). "Benefits of Physical Training in Sarcoidosis". Lung. 193 (5): 701–8. doi:10.1007/s00408-015-9784-9. PMID 26286208.
- Karadallı MN, Boşnak-Güçlü M, Camcıoğlu B, Kokturk N, Türktaş H (April 2016). "Effects of Inspiratory Muscle Training in Subjects With Sarcoidosis: A Randomized Controlled Clinical Trial". Respiratory Care. 61 (4): 483–94. doi:10.4187/respcare.04312. PMID 26715771.
- Spruit MA, Wouters EF, Gosselink R (2005). Rehabilitation programmes in sarcoidosis: a multidisciplinary approach. European Respiratory Monograph. 32. pp. 316–326. doi:10.1183/1025448x.00032021. ISBN 9781904097372.
- Baughman RP, Nunes H (February 2013). "Sarcoidosis-associated fatigue: an often forgotten symptom--author reply". Expert Review of Clinical Immunology. 9 (2): 111. doi:10.1586/ECI.12.93. PMID 23390941.
- "What Is Sarcoidosis?". National Heart, Lung and Blood Institute. National Institutes of Health. 14 June 2013. Archived from the original on 27 February 2014. Retrieved 21 February 2014.
- Syed J, Myers R (January 2004). "Sarcoid heart disease". The Canadian Journal of Cardiology. 20 (1): 89–93. PMID 14968147.
- Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB (September 2013). "Corticosteroid therapy for cardiac sarcoidosis: a systematic review". The Canadian Journal of Cardiology. 29 (9): 1034–41. doi:10.1016/j.cjca.2013.02.004. PMID 23623644.
- Rossides M, Kullberg S, Askling J, Eklund A, Grunewald J, Arkema EV (February 2018). "Sarcoidosis mortality in Sweden: a population-based cohort study". The European Respiratory Journal. 51 (2): 1701815. doi:10.1183/13993003.01815-2017. PMC 5886843. PMID 29467203.
- Karakantza M, Matutes E, MacLennan K, O'Connor NT, Srivastava PC, Catovsky D (March 1996). "Association between sarcoidosis and lymphoma revisited". Journal of Clinical Pathology. 49 (3): 208–12. doi:10.1136/jcp.49.3.208. PMC 500399. PMID 8675730.
- Askling J, Grunewald J, Eklund A, Hillerdal G, Ekbom A (November 1999). "Increased risk for cancer following sarcoidosis". American Journal of Respiratory and Critical Care Medicine. 160 (5 Pt 1): 1668–72. doi:10.1164/ajrccm.160.5.9904045. PMID 10556138.
- Tana C, Giamberardino MA, Di Gioacchino M, Mezzetti A, Schiavone C (April–June 2013). "Immunopathogenesis of sarcoidosis and risk of malignancy: a lost truth?". International Journal of Immunopathology and Pharmacology. 26 (2): 305–13. doi:10.1177/039463201302600204. PMID 23755746.
- Kornacker M, Kraemer A, Leo E, Ho AD (February 2002). "Occurrence of sarcoidosis subsequent to chemotherapy for non-Hodgkin's lymphoma: report of two cases". Annals of Hematology. 81 (2): 103–5. doi:10.1007/s00277-001-0415-6. PMID 11907791.
- Suvajdzic N, Milenkovic B, Perunicic M, Stojsic J, Jankovic S (2007). "Two cases of sarcoidosis-lymphoma syndrome". Medical Oncology. 24 (4): 469–71. doi:10.1007/s12032-007-0026-8. PMID 17917102.
- London J, Grados A, Fermé C, Charmillon A, Maurier F, Deau B, Crickx E, Brice P, Chapelon-Abric C, Haioun C, Burroni B, Alifano M, Le Jeunne C, Guillevin L, Costedoat-Chalumeau N, Schleinitz N, Mouthon L, Terrier B (November 2014). "Sarcoidosis occurring after lymphoma: report of 14 patients and review of the literature". Medicine. 93 (21): e121. doi:10.1097/MD.0000000000000121. PMC 4616278. PMID 25380084.
- Yao M, Funk GF, Goldstein DP, DeYoung BR, Graham MM (January 2005). "Benign lesions in cancer patients: Case 1. Sarcoidosis after chemoradiation for head and neck cancer". Journal of Clinical Oncology. 23 (3): 640–1. doi:10.1200/JCO.2005.02.089. PMID 15659510.
- Yamasawa H, Ishii Y, Kitamura S (2000). "Concurrence of sarcoidosis and lung cancer. A report of four cases". Respiration; International Review of Thoracic Diseases. 67 (1): 90–3. doi:10.1159/000029470. PMID 10705270.
- Zambrana F, Antúnez A, García-Mata J, Mellado JM, Villar JL (June 2009). "Sarcoidosis as a diagnostic pitfall of pancreatic cancer". Clinical & Translational Oncology. 11 (6): 396–8. doi:10.1007/s12094-009-0375-1. PMID 19531456.
- Schiller G, Said J, Pal S (October 2003). "Hairy cell leukemia and sarcoidosis: a case report and review of the literature". Leukemia. 17 (10): 2057–9. doi:10.1038/sj.leu.2403074. PMID 14513061.
- Maloisel F, Oberling F (January 1992). "Acute myeloid leukemia complicating sarcoidosis". Journal of the Royal Society of Medicine. 85 (1): 58–9. PMC 1293471. PMID 1548666.
- Reich JM (January 1985). "Acute myeloblastic leukemia and sarcoidosis. Implications for pathogenesis". Cancer. 55 (2): 366–9. doi:10.1002/1097-0142(19850115)55:2<366::AID-CNCR2820550212>3.0.CO;2-1. PMID 3855267.
- Jamilloux Y, Valeyre D, Lortholary O, Bernard C, Kerever S, Lelievre L, Neel A, Broussolle C, Seve P (January 2015). "The spectrum of opportunistic diseases complicating sarcoidosis". Autoimmunity Reviews. 14 (1): 64–74. doi:10.1016/j.autrev.2014.10.006. PMID 25305373.
- Jamilloux Y, Néel A, Lecouffe-Desprets M, Fèvre A, Kerever S, Guillon B, Bouvry D, Varron L, Redares C, Dominique S, Roux M, Chapelon-Abric C, Valeyre D, Ducray F, Bernard C, Broussolle C, Hamidou M, Sève P (April 2014). "Progressive multifocal leukoencephalopathy in patients with sarcoidosis". Neurology. 82 (15): 1307–13. doi:10.1212/WNL.0000000000000318. PMID 24610328.
- Sam, Amir H.; James T.H. Teo (2010). Rapid Medicine. Wiley-Blackwell. ISBN 978-1405183239.
- Henke CE, Henke G, Elveback LR, Beard CM, Ballard DJ, Kurland LT (May 1986). "The epidemiology of sarcoidosis in Rochester, Minnesota: a population-based study of incidence and survival" (PDF). American Journal of Epidemiology. 123 (5): 840–5. doi:10.1093/oxfordjournals.aje.a114313. PMID 3962966.
- Rutherford RM, Brutsche MH, Kearns M, Bourke M, Stevens F, Gilmartin JJ (September 2004). "Prevalence of coeliac disease in patients with sarcoidosis". European Journal of Gastroenterology & Hepatology. 16 (9): 911–5. doi:10.1097/00042737-200409000-00016. PMID 15316417.
- Baughman RP, Lower EE, du Bois RM (March 2003). "Sarcoidosis". Lancet. 361 (9363): 1111–8. doi:10.1016/S0140-6736(03)12888-7. PMID 12672326.
- Lazarus A (November 2009). "Sarcoidosis: epidemiology, etiology, pathogenesis, and genetics". Disease-A-Month. 55 (11): 649–60. doi:10.1016/j.disamonth.2009.04.008. PMID 19857640.
- Sharma, OP (2005). "Chapter 1: Definition and history of sarcoidosis". Sarcoidosis. Sheffield: European Respiratory Society Journals. ISBN 9781904097884.
- Babalian, L (26 January 1939). "Disease of Besnier-Boeck-Schaumann". New England Journal of Medicine. 220 (4): 143–145. doi:10.1056/NEJM193901262200404.
- "Join WASOG". wasog.org. World Association of Sarcoidosis and Other Granulomatous Disorders. Archived from the original on 1 February 2014. Retrieved 21 February 2014.
- "Index". Sarcoidosis, Vasculitis and Diffuse Lung Diseases. 2016. Archived from the original on 6 May 2016. Retrieved 9 April 2016.
- "Mission & Goals". Foundation for Sarcoidosis Research. Archived from the original on 26 February 2014. Retrieved 21 February 2014.
- Izbicki G, Chavko R, Banauch GI, Weiden MD, Berger KI, Aldrich TK, Hall C, Kelly KJ, Prezant DJ (May 2007). "World Trade Center "sarcoid-like" granulomatous pulmonary disease in New York City Fire Department rescue workers" (PDF). Chest. 131 (5): 1414–23. doi:10.1378/chest.06-2114. PMID 17400664.
- "9/11 Health – What We Know About the Health Effects of 9/11". NYC. US Government. Archived from the original on 28 January 2014. Retrieved 22 February 2014.
- Grimes, William (10 August 2008). "Bernie Mac, Acerbic Stand-Up Comedian and Irascible TV Dad, Dies at 50". The New York Times. Archived from the original on 14 March 2014. Retrieved 30 April 2014.
- Le Mignot, Suzanne (August 9, 2008). "Actor and comedian Bernie Mac dies at age 58". CBS2Chicago. Archived from the original on October 21, 2009. Retrieved 2010-03-27.
- Kat Carney (September 19, 2003). Former MTV VJ tells of battle with chronic illness CNN.com, accessed 10 August 2019
- "Thursday roundup: Maddox rides to Ben's defense". May 20, 2005. Retrieved January 30, 2017.
- Donna J. Miller (????) Coroner says singer Sean Levert died of natural causes. Cleveland.com, accessed 11 August 2019
- Zolan Kanno-Youngs. "Wall Street Journal's Joseph Rago Died of Natural Causes, Medical Examiner Says". Wall Street Journal. Retrieved 12 September 2017.
- Charlier P, Froesch P (December 2013). "Robespierre: the oldest case of sarcoidosis?". Lancet. 382 (9910): 2068. doi:10.1016/S0140-6736(13)62694-X. PMID 24360387.
- Mai FM (2006). "Beethoven's terminal illness and death". The Journal of the Royal College of Physicians of Edinburgh. 36 (3): 258–63. PMID 17214130.
- Sharma OP (2005). "Murray Kornfeld, American College of Chest Physician, and sarcoidosis: a historical footnote: 2004 Murray Kornfeld Memorial Founders Lecture". Chest. 128 (3): 1830–35. doi:10.1378/chest.128.3.1830. PMID 16162793.
- Subramanian P, Chinthalapalli H, Krishnan M, Tarlo SM, Lobbedez T, Pineda ME, Oreopoulos DG (September 2004). "Pregnancy and sarcoidosis: an insight into the pathogenesis of hypercalciuria". Chest. 126 (3): 995–8. doi:10.1378/chest.126.3.995. PMID 15364785.
External links
| Classification | |
|---|---|
| External resources |
- Sarcoidosis UK Information Hub
- Iannuzzi, M. C.; Sah, B. P. (March 2008). Sarcoidosis: Interstitial Lung Diseases. The Merck Manual Home Edition. Merck Sharp & Dohme Corp. Retrieved 19 February 2014.