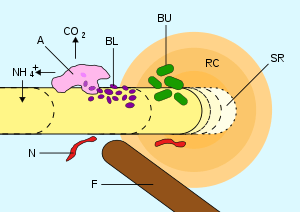
Rhizosphere
The rhizosphere is the narrow region of soil that is directly influenced by root secretions, and associated soil microorganisms known as the root microbiome.[2] The rhizosphere contains many bacteria and other microorganisms that feed on sloughed-off plant cells, termed rhizodeposition,[3] and the proteins and sugars released by roots. This symbiosis leads to more complex interactions, influencing plant growth and competition for resources. Much of the nutrient cycling and disease suppression needed by plants occurs immediately adjacent to roots due to root exudants and communities of microorganisms.[4] The rhizosphere also provides space to produce allelochemicals to control neighbours and relatives.[5] The plant-soil feedback loop and other physical factors are important selective pressures for the communities and growth in the rhizosphere.
Chemical interactions
Chemical availability
Exudates, such as organic acids, change the chemical structure of the rhizosphere in comparison with the bulk soil. Concentrations of organic acids and saccharides affect the ability of the plant to uptake phosphorus, nitrogen,[6][7] potassium and water through the root cap,[8] and the total availability of iron to the plant and to its neighbors.[9] The ability of the plant to affect the availability of iron and other minerals for its neighbors by providing specific transport proteins affects the composition of the community and fitness.
Exudates come in the form of chemicals released into the rhizosphere by cells in the roots and cell waste referred to as "rhizodeposition." This rhizodeposition comes in various forms of organic carbon and nitrogen that provide for the communities around plant roots and dramatically affect the chemistry surrounding the roots.[10] Exopolysaccharides, such as PGA, affect the ability of roots to uptake water by maintaining the stability of the rhizosphere and controlling the flow of water.[11] In potato cultivars, phenols and lignins comprise the greatest number of ion influencing compounds produced as plant exudates regardless of location; however, the intensity of different compounds was found to be influenced by soils and environmental conditions, resulting in variation amongst nitrogen compounds, lignins, phenols, carbohydrates, and amines.[12]
Allelochemicals
Chemicals connected to allelopathy: flavinols [13] carbohydrates and application by root hairs[14] phenols[15] Positive allelopathic pathways and definitions of interactions between plant-plant and plant-microbe,[16] positive plant-microbe in the form of systematic resistance[17]
Although it goes beyond the rhizosphere area, it is notable that some plants secrete allelochemicals from their roots which inhibit the growth of other organisms. For example, garlic mustard produces a chemical which is believed to prevent mutualisms forming between the surrounding trees and mycorrhiza in mesic North American temperate forests where it is an invasive species.[18]
Ecology of the rhizosphere
Rhizodeposition allows for the growth of communities of microorganisms directly surrounding and inside plant roots. This leads to complex interactions between species including mutualism, predation/parasitism, and competition.
Predation
Predation is considered to be top-down because these interactions decrease the population, but the closeness of the interactions of species directly affects the availability of resources causing the population to also be affected by bottom-up controls.[19] Without soil fauna, microbes that directly prey upon competitors of plants and plant mutualists, interactions within the rhizosphere would be antagonistic toward the plants. Soil fauna provide the top-down component of the rhizosphere while also allowing for the bottom-up increase in nutrients from rhizodeposition and inorganic nitrogen. The complexity of these interactions has also been shown through experiments with common soil fauna, such as nematodes and protists. Predation by bacterial-feeding nematodes was shown to influence nitrogen availability and plant growth.[20] There was also an increase in the populations of bacteria to which nematodes were added. Predation upon Pseudomonas by amoeba shows predators are able to upregulate toxins produced by prey without direct interaction using supernatant.[21] The ability of predators to control the expression and production of biocontrol agents in prey without direct contact is related to the evolution of prey species to signals of high predator density and nutrient availability.
The food web in the rhizosphere can be considered as three different channels with two different sources of energy: the detritus-dependent channels are fungi and bacterial species, and the root energy dependent channel consists of nematodes, symbiotic species, and some arthropods.[19] This food web is constantly in flux, since the amount of detritus available and the rate of root sloughing changes as roots grow and age. This bacterial channel is considered to be a faster channel because of the ability of species to focus on more accessible resources in the rhizosphere and have faster regeneration times compared with the fungal channel. All three of these channels are also interrelated to the roots that form the base of the rhizosphere ecosystem and the predators, such as the nematodes and protists, that prey upon many of the same species of microflora.
Competition
Allelopathy and autotoxicity and negative root-root communications[22][9]
The competition between plants due to released exudates is dependent geometrical properties, which determine the capacity of interception of exudates from any point in relation to the plants’ roots, and physicochemical properties, which determine the capacity of each root to take up exudates in the area.[23] Geometrical properties are the density of roots, root diameter, and distribution of the roots and physicochemical properties are exudation rate, decay rate of exudates, and the properties of the environment that affect diffusion. These properties define the rhizosphere of roots and the likelihood that plants can directly compete with neighbors.
Plants and soil microflora indirectly compete against one another by tying up limiting resources, such as carbon and nitrogen, into their biomass.[24] This competition can occur at varying rates due to the ratio of carbon to nitrogen in detritus and the ongoing mineralization of nitrogen in the soil. Mycorrhizae and heterotrophic soil microorganisms compete for both carbon and nitrogen depending upon which is limiting at the time, which itself heavily depends on the species, scavenging abilities, and the environmental conditions affecting nitrogen input. Plants are less successful at uptake of organic nitrogen, such as amino acids, than the soil microflora that exist in the rhizosphere.[25] This informs other mutualistic relationships formed by plants with relation to nitrogen uptake.
Competition over other resources, such as oxygen in limited environments, are directly affected by the spatial and temporal locations of species and the rhizosphere. In methanotrophs, proximity to higher density roots and the surface are important and help to determine where they are dominant over heterotrophs in rice paddies.[26]
The weak connection between the various channels of energy is important in regulation in the populations of both predator and prey and the availability of resources to the biome. Strong connections between resource-consumer and consumer-consumer create coupled systems of oscillators which are then determined by the nature of the available resources.[27] These systems can then be considered cyclical, quasi-periodic, or chaotic.
Mutualism
Plants secrete many compounds through their roots to serve symbiotic functions in the rhizosphere. Strigolactones, secreted and detected by mycorrhizal fungi, stimulate the germination of spores and initiate changes in the mycorrhiza that allow it to colonize the root. The parasitic plant, Striga also detects the presence of strigolactones and will germinate when it detects them; they will then move into the root, feeding off the nutrients present.[28][29]
Symbiotic nitrogen-fixing bacteria, such as Rhizobium species, detect compounds like flavonoids secreted by the roots of leguminous plants and then produce nod factors which signal to the plant that they are present and will lead to the formation of root nodules. In these nodules bacteria, sustained by nutrients from the plant, convert nitrogen gas to a form that can be used by the plant.[30] Non-symbiotic (or "free-living") nitrogen-fixing bacteria may reside in the rhizosphere just outside the roots of certain plants (including many grasses), and similarly "fix" nitrogen gas in the nutrient-rich plant rhizosphere. Even though these organisms are thought to be only loosely associated with plants they inhabit, they may respond very strongly to the status of the plants. For example, nitrogen-fixing bacteria in the rhizosphere of the rice plant exhibit diurnal cycles that mimic plant behavior, and tend to supply more fixed nitrogen during growth stages when the plant exhibits a high demand for nitrogen.[31]
In exchange for the resources and shelter that plants and roots provide, fungi and bacteria control pathogenic microbes.[32] The fungi that perform such activities also serve close relationships with species of plants in the form of mycorrhizal fungi, which are diverse in how they relate to plants. Arbuscular mycorrhizal fungi and the bacteria that make the rhizosphere their home also form close relationships in order to be more competitive.[33] which plays into the bigger cycles of nutrients that impact the ecosystem, such as biogeochemical pathways.[9]
Community structure
Bais et al. (2004) call the rhizosphere an information super highway because of the proximity of data points, which include roots and other organisms in the soil, and the methods for transferring data using exudates and communities.[22] This description has been used to explain the complex interactions that plants, their fungal mutualists, and the bacterial species that live in the rhizosphere have entered into over the course of their evolution. Certain species like Trichoderma are interesting because of their ability to select for species in this complex web. Trichoderma is a biological control agent because of evidence that it can reduce plant pathogens in the rhizosphere.[34] Plants themselves also impact which bacterial species are in the rhizosphere which are selected against because of the introduction of exudates and the relationships that it maintains. The control of which species are in these small diversity hotspots can drastically affect the capacity of these spaces and future conditions for future ecologies.[32][5]
Methods
The following are methods that are commonly used or of interest to the topics discussed in this article. Many of these methods include both field testing of the root systems and in lab testing using simulated environments to perform experiments, such as pH determination.[35]
- High-throughput screening
- Mini rhizotron camera
- Various methods used to determine water movement in the rhizosphere e.g. microelectrodes and agar techniques for pH and microsampling of rhizosphere materials[35]
- Pyrolysis–field ionization mass spectrometry allows for spectrometry of agricultural fields to find fulvic and humic acids and the extraction residues (humins) in certain studies and expanded to general organic compounds in other recent work.[36][12]
See also
- Soil respiration
- Allelopathy
- Bulk soil
- Root mucilage
- Mycorrhiza
References
- Giri, B.; Giang, P. H.; Kumari, R.; Prasad, R.; Varma, A. (2005). "Microbial Diversity in Soils". Microorganisms in Soils: Roles in Genesis and Functions. Soil Biology. 3. pp. 19–55. doi:10.1007/3-540-26609-7_2. ISBN 978-3-540-22220-0.
- "Microbial Health of the Rhizosphere". Archived from the original on March 12, 2007. Retrieved 5 May 2006.
- Hütsch, Birgit W.; Augustin, Jürgen; Merbach, Wolfgang (2002). "Plant rhizodeposition – an important source for carbon turnover in soils". Journal of Plant Nutrition and Soil Science. 165 (4): 397–407. doi:10.1002/1522-2624(200208)165:4<397::AID-JPLN397>3.0.CO;2-C – via Research Gate.
- Elaine R. Ingham. "The Soil Food Web". USDA-NRCS. Retrieved 3 July 2006.
- Grayston, Susan J.; Wang, Shenquiang; Campbell, Colin D.; Edwards, Anthony C. (March 1998). "Selective influence of plant species on microbial diversity in the rhizosphere". Soil Biology and Biochemistry. 30 (3): 369–378. doi:10.1016/S0038-0717(97)00124-7.
- Jones, David L. (August 1998). "Organic acids in the rhizosphere – a critical review". Plant and Soil. 205 (1): 25–44. doi:10.1023/A:1004356007312.
- Hinsinger, Philippe (December 2001). "Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review". Plant and Soil. 237 (2): 173–195. doi:10.1023/A:1013351617532.
- Walker, Travis S.; Bais, Harsh Pal; Grotewold, Erich; Vivanco, Jorge M. (May 2003). "Root Exudation and Rhizosphere Biology". Plant Physiol. 132 (1): 44–51. doi:10.1104/pp.102.019661. PMC 1540314. PMID 12746510.
- Lambers, Hans; Mougel, Christophe; Jaillard, Benoît; Hinsinger, Philippe (August 2009). "Plant-microbe-soil interactions in the rhizosphere: an evolutionary perspective". Plant Soil. 321 (1–2): 83–115. doi:10.1007/s11104-009-0042-x.
- Hinsinger, Philippe; Gobran, George R.; Gregory, Peter J.; Wenzel, Walter W. (November 2005). "Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes". New Phytologist. 168 (2): 293–303. doi:10.1111/j.1469-8137.2005.01512.x. ISSN 1469-8137. PMID 16219069.
- Czarnes, S.; Hallett, P. D.; Bengough, A. G.; Young, I. M. (2000). "Root- and microbial-derived mucilages affect soil structure and water transport". European Journal of Soil Science. 51 (3): 435. doi:10.1046/j.1365-2389.2000.00327.x.
- Schlichting, André; Leinweber, Peter (2009). "New evidence for the molecular–chemical diversity of potato plant rhizodeposits obtained by pyrolysis–field Ionisation mass spectrometry". Phytochemical Analysis. 20 (1): 1–13. doi:10.1002/pca.1080. ISSN 1099-1565. PMID 18618895.
- Leslie A. Weston & Ulrike Mathesius (2013). "Flavonoids: Their Structure, Biosynthesis and Role in the Rhizosphere, Including Allelopathy". Journal of Chemical Ecology. 39 (2): 283–297. doi:10.1007/s10886-013-0248-5. PMID 23397456.
- Cecile Bertin, Xiaohan Yang, Leslie A. Weston (2003). "The role of root exudates and allelochemicals in the rhizosphere". Plant and Soil. 256: 67–83. doi:10.1023/A:1026290508166.CS1 maint: multiple names: authors list (link)
- Glass, Anthony D. M. (1976). "The allelopathic potential of phenolic acids associated with the rhizosphere of Pteridium aquilinum". Canadian Journal of Botany. 54 (21): 2440–2444. doi:10.1139/b76-259.
- Bais, Harsh P.; Weir, Tiffany L.; Perry, Laura G.; Gilroy, Simon; Vivanco, Jorge M. (2006). "The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms". Annual Review of Plant Biology. 57 (1): 233–266. doi:10.1146/annurev.arplant.57.032905.105159. PMID 16669762.
- van Loon, L. C.; Bakker, P. A. H. M.; Pieterse, C. M. J. (28 November 2003). "Systemic Resistance Induced by Rhizosphere Bacteria". Annual Review of Phytopathology. 36 (1): 453–483. doi:10.1146/annurev.phyto.36.1.453. hdl:1874/7701. PMID 15012509.
- Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC, Hallett SG, Prati D, Klironomos JN; Campbell; Powell; Wolfe; Callaway; Thelen; Hallett; Prati; Klironomos (2006). "Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms". PLoS Biology. 4 (5): e140. doi:10.1371/journal.pbio.0040140. PMC 1440938. PMID 16623597.CS1 maint: multiple names: authors list (link)
- Moore, John C.; McCann, Kevin; Setälä, Heikki; De Ruiter, Peter C. (2003). "Top-Down is Bottom-Up: Does Predation in the Rhizosphere Regulate Aboveground Dynamics?". Ecology. 84 (4): 846. doi:10.1890/0012-9658(2003)084[0846:TIBDPI]2.0.CO;2.
- Ingham, Russell E.; Trofymow, J. A.; Ingham, Elaine R.; Coleman, David C. (1985). "Interactions of Bacteria, Fungi, and their Nematode Grazers: Effects on Nutrient Cycling and Plant Growth". Ecological Monographs. 55 (1): 119–140. doi:10.2307/1942528. JSTOR 1942528.
- Jousset, Alexandre; Rochat, Laurène; Scheu, Stefan; Bonkowski, Michael; Keel, Christoph (August 2010). "Predator-Prey Chemical Warfare Determines the Expression of Biocontrol Genes by Rhizosphere-Associated Pseudomonas fluorescens". Appl. Environ. Microbiol. 76 (15): 5263–5268. doi:10.1128/AEM.02941-09. PMC 2916451. PMID 20525866.
- Bais, Harsh Pal; Park, Sang-Wook; Weir, Tiffany L; Callaway, Ragan M; Vivanco, Jorge M (2004). "How plants communicate using the underground information superhighway". Trends in Plant Science. 9 (1): 26–32. doi:10.1016/j.tplants.2003.11.008. PMID 14729216.
- Raynaud, Xavier; Jaillard, Benoît; Leadley, Paul W. (2008). "Plants May Alter Competition by Modifying Nutrient Bioavailability in Rhizosphere: A Modeling Approach" (PDF). The American Naturalist. 171 (1): 44–58. doi:10.1086/523951. ISSN 0003-0147. PMID 18171150.
- Kaye, Jason P.; Hart, Stephen C. (1997). "Competition for nitrogen between plants and soil microorganisms". Trends in Ecology & Evolution. 12 (4): 139–43. doi:10.1016/S0169-5347(97)01001-X. PMID 21238010.
- Owen, A.G; Jones, D.L (2001). "Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition". Soil Biology and Biochemistry. 33 (4–5): 651–657. doi:10.1016/s0038-0717(00)00209-1.
- Bodegom, Peter van; Stams, Fons; Mollema, Liesbeth; Boeke, Sara; Leffelaar, Peter (August 2001). "Methane Oxidation and the Competition for Oxygen in the Rice Rhizosphere". Appl. Environ. Microbiol. 67 (8): 3586–3597. doi:10.1128/AEM.67.8.3586-3597.2001. PMC 93059. PMID 11472935.
- McCann, Kevin; Hastings, Alan; Huxel, Gary R. (22 October 1998). "Weak trophic interactions and the balance of nature". Nature. 395 (6704): 794–798. doi:10.1038/27427.
- Arnaud Besserer, Virginie Puech-Pagès, Patrick Kiefer, Victoria Gomez-Roldan, Alain Jauneau, Sébastien Roy, Jean-Charles Portais, Christophe Roux, Guillaume Bécard, Nathalie Séjalon-Delmas (2006). "Strigolactones Stimulate Arbuscular Mycorrhizal Fungi by Activating Mitochondria". PLOS Biology. 4 (7): e226. doi:10.1371/journal.pbio.0040226. PMC 1481526. PMID 16787107.CS1 maint: multiple names: authors list (link)
- Andreas Brachmann, Martin Parniske (2006). "The Most Widespread Symbiosis on Earth". PLOS Biology. 4 (7): e239. doi:10.1371/journal.pbio.0040239. PMC 1489982. PMID 16822096.
- Tian, C. F.; Garnerone, A.-M.; Mathieu-Demaziere, C.; Masson-Boivin, C.; Batut, J. (2012). "Plant-activated bacterial receptor adenylate cyclases modulate epidermal infection in the Sinorhizobium meliloti-Medicago symbiosis". Proceedings of the National Academy of Sciences. 109 (17): 6751–6756. doi:10.1073/pnas.1120260109. PMC 3340038. PMID 22493242.
- Sims GK, Dunigan EP; Dunigan (1984). "Diurnal and seasonal variations in nitrogenase activity (C2H2 reduction) of rice roots". Soil Biology and Biochemistry. 16 (1): 15–18. doi:10.1016/0038-0717(84)90118-4.
- Weller, D. M. (1988). "Biological Control of Soilborne Plant Pathogens in the Rhizosphere with Bacteria". Annual Review of Phytopathology. 26 (1): 379–407. doi:10.1146/annurev.py.26.090188.002115.
- Bianciotto, V.; Minerdi, D.; Perotto, S.; Bonfante, P. (1996). "Cellular interactions between arbuscular mycorrhizal fungi and rhizosphere bacteria". Protoplasm. 193 (1–4): 123–131. doi:10.1007/BF01276640.
- Howell, C. R. (2003). "Mechanisms Employed by Trichoderma Species in the Biological Control of Plant Diseases: The History and Evolution of Current Concepts". Plant Disease. 87: 4–10. doi:10.1094/PDIS.2003.87.1.4.
- Gregory, P.J.; Hinsinger, P. (1999). "New approaches to studying chemical and physical changes in the rhizosphere: an overview". Plant and Soil. 211: 1–9. doi:10.1023/A:1004547401951.
- Schlten, Hans-Rolf; Leinweber, Peter (April 1993). "Pyrolysis-field ionization mass spectrometry of agricultural soils and humic substances: Effect of cropping systems and influence of the mineral matrix". Plant and Soil. 151 (1): 77–90. doi:10.1007/BF00010788. ISSN 0032-079X.
Further reading
- "The Soil Habitat". University of Western Australia. Archived from the original on 20 August 2006. Retrieved 3 July 2006.
- Andrew Wylie (2006). "Digging in the Dirt: Is the Study of the Rhizosphere Ripe for a Systems Biology Approach?". Science Creative Quarterly. Retrieved 24 April 2017.
- McNear Jr., D. H. (2013). "The Rhizosphere – Roots, Soil and Everything In Between". Nature Education Knowledge. 4 (3): 1. Retrieved 24 April 2017.