Rabacfosadine
Rabacfosadine, marketed by VetDC under the brand name Tanovea-CA1 (rabacfosadine for injection), is a guanine nucleotide analog used for the treatment of lymphoma in dogs. The drug is conditionally approved by the U.S. Food and Drug Administration under application number 141-475 for use in treating canine lymphoma[1] pending a full demonstration of effectiveness. Originally developed by Gilead Sciences as GS-9219,[2] rabacfosadine is no longer being pursued for use in the treatment of lymphoma in humans.
 | |
| Clinical data | |
|---|---|
| Trade names | TANOVEA-CA1 |
| AHFS/Drugs.com | Veterinary Use |
| Routes of administration | Intravenous |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
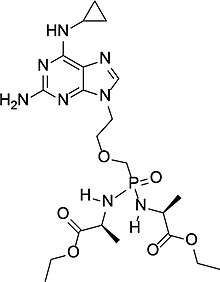
| Formula | C11H35N8O6P |
| Molar mass | 406.425 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The active form of rabacfosadine is a chain-terminating inhibitor of the major deoxyribonucleic acid (DNA) polymerases. In vitro studies have demonstrated that rabacfosadine inhibits DNA synthesis, resulting in S phase arrest and induction of apoptosis.[3] It also inhibits the proliferation of lymphocytes in dogs with naturally occurring lymphoma.[4]
Veterinary uses
In canine lymphoma, rabacfosadine can be used both in dogs new to treatment and in dogs that are no longer responding to conventional chemotherapy. It comes in the form of a sterile, lyophilized powder that is reconstituted and administered as a 30-minute intravenous infusion. Infusions are given once every three weeks for up to five treatments.[5]
Adverse effects
Common side effects of rabacfosadine are decreased white blood cell count, diarrhea, vomiting, decreased appetite or loss of appetite, weight loss, decreased activity level, and skin problems. Other side effects may occur.[1]
References
- "FDA Conditionally Approves Tanovea-CA1, the first New Animal Drug for Treating Lymphoma in Dogs". U.S. Food and Drug Administration. January 3, 2017.
- De Clercq, Erik (2018-08-01). "Tanovea® for the treatment of lymphoma in dogs". Biochemical Pharmacology. 154: 265–269. doi:10.1016/j.bcp.2018.05.010. ISSN 0006-2952. PMID 29778492.
- Tumas, Daniel B.; Jeraj, Robert; Thamm, Douglas H.; Vail, David; Plunkett, William; Lee, William A.; Fridland, Arnold; Rhodes, Gerald R.; Desai, Manoj (2008-05-01). "GS-9219—A Novel Acyclic Nucleotide Analogue with Potent Antineoplastic Activity in Dogs with Spontaneous Non–Hodgkin's Lymphoma". Clinical Cancer Research. 14 (9): 2824–2832. doi:10.1158/1078-0432.CCR-07-2061. ISSN 1078-0432. PMID 18451250.
- Lawrence, Jessica; Vanderhoek, Matthew; Barbee, David; Jeraj, Robert; Tumas, Daniel B.; Vail, David M. (2009). "Use of 3′-Deoxy-3′-[18f]fluorothymidine Pet/Ct for Evaluating Response to Cytotoxic Chemotherapy in Dogs with Non-Hodgkin's Lymphoma". Veterinary Radiology & Ultrasound. 50 (6): 660–668. doi:10.1111/j.1740-8261.2009.01612.x. ISSN 1740-8261.
- "Canine Lymphoma Treatment | TANOVEA For Pet Owners". VetDC. Retrieved 2019-10-02.