Pyronaridine
Pyronaridine is an antimalarial drug.[1] It was first made in 1970 and has been in clinical use in China since the 1980s.[2]
 | |
| Clinical data | |
|---|---|
| Other names | Pyronaridine tetraphosphate |
| Routes of administration | Oral, intramuscular injection, intravenous therapy |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
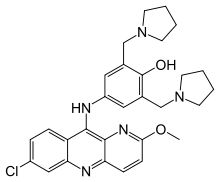
| Formula | C29H32ClN5O2 |
| Molar mass | 518.06 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
It is one of the components of the artemisinin combination therapy pyronaridine/artesunate (Pyramax).[3]
It has also been studied as a potential anticancer drug.[4]
References
- Croft, Simon L.; Duparc, Stephan; Arbe-Barnes, Sarah J.; Craft, J.; Shin, Chang-Sik; Fleckenstein, Lawrence; Borghini-Fuhrer, Isabelle; Rim, Han-Jong (2012). "Review of pyronaridine anti-malarial properties and product characteristics". Malaria Journal. 11: 270. doi:10.1186/1475-2875-11-270. PMC 3483207. PMID 22877082.
- Chang C, Lin-Hua T, Jantanavivat C (1992). "Studies on a new antimalarial compound: pyronaridine". Trans R Soc Trop Med Hyg. 86 (1): 7–10. doi:10.1016/0035-9203(92)90414-8. PMID 1566313.
- "Pyramax" (PDF). European Medicines Agency. 2016.
- Villanueva, Paulina J.; Martinez, Alberto; Baca, Sarah T.; Dejesus, Rebecca E.; Larragoity, Manuel; Contreras, Lisett; Gutierrez, Denisse A.; Varela-Ramirez, Armando; Aguilera, Renato J. (2018). "Pyronaridine exerts potent cytotoxicity on human breast and hematological cancer cells through induction of apoptosis". PLOS ONE. 13 (11): e0206467. doi:10.1371/journal.pone.0206467. PMC 6218039. PMID 30395606.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.