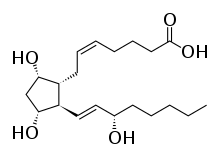
Prostaglandin F2alpha
Prostaglandin F2α (PGF2α in prostanoid nomenclature), pharmaceutically termed carboprost, is a naturally occurring prostaglandin used in medicine to induce labor and as an abortifacient.[1]
 | |
| Clinical data | |
|---|---|
| Other names | Amoglandin, Croniben, Cyclosin, Dinifertin, Enzaprost, Glandin, PGF2α, Panacelan, Prostamodin |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intravenous (cannot used to induce labor)because it cannot be used in cervix, intra-amniotic (to induce abortion) |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 3 to 6 hours in amniotic fluid, less than 1 minute in blood plasma |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.209.720 |
| Chemical and physical data | |
| Formula | C20H34O5 |
| Molar mass | 354.48 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | 200 mg/mL (20 °C) |
SMILES
| |
InChI
| |
| | |
In domestic mammals, it is produced by the uterus when stimulated by oxytocin, in the event that there has been no implantation during the luteal phase. It acts on the corpus luteum to cause luteolysis, forming a corpus albicans and stopping the production of progesterone. Action of PGF2α is dependent on the number of receptors on the corpus luteum membrane.
The PGF2α isoform 8-iso-PGF2α was found in significantly increased amounts in patients with endometriosis, thus being a potential causative link in endometriosis-associated oxidative stress.[2]
Mechanism of action
PGF2α acts by binding to the prostaglandin F2α receptor. It is released in response to an increase in oxytocin levels in the uterus, and stimulates both luteolytic activity and the release of oxytocin.[3] Because PGF2α is linked with an increase in uterine oxytocin levels, there is evidence that PGF2α and oxytocin form a positive feedback loop to facilitate the degradation of the corpus luteum.[4] PGF2α and oxytocin also inhibit the production of progesterone, a hormone that facilitates corpus luteum development. Conversely, higher progesterone levels inhibit production of PGF2α and oxytocin, as the effects of the hormones are in opposition to each other.
Pharmaceutical Use
When injected into the body or amniotic sac, PGF2α can either induce labor or cause an abortion depending on the concentration used. In small doses (1–4 mg/day), PGF2α acts to stimulate uterine muscle contractions, which aids in the birth process. However, during the first trimester and in higher concentrations (40 mg/day),[5] PGF2α can cause an abortion by degrading the corpus luteum, which nourishes the fetus in the womb. Since the fetus is not viable outside the womb by this time, the lack of sustenance starves and aborts the fetus after a day or two.
Synthesis
Industrial Synthesis
In 2012 a concise and highly stereoselective total synthesis of PGF2α was described.[6] The synthesis requires only seven steps, a huge improvement on the original 17-steps synthesis of Corey and Cheng,[7] and uses 2,5-dimethoxytetrahydrofuran as a starting reagent, with S-proline as an asymmetric catalyst.
Biosynthesis
In the body PGF2α is synthesized in several distinct steps. First, Phospholipase A2 (PLA2) facilitates the conversion of phospholipids to Arachidonic Acid, the framework from which all prostaglandins are formed.[8] The Arachidonic Acid then reacts with two Cyclooxygenase (COX) receptors, COX-1 and COX-2 to form Prostaglandin H2, an intermediate. Lastly, the compound reacts with Aldose Reductase (AKR1B1) to form PGF2α.[8]
Analogues
The following medications are analogues of prostaglandin F2α:
References
- The Merck index : an encyclopedia of chemicals, drugs, and biologicals. O'Neil, Maryadele J., Royal Society of Chemistry (Great Britain) (15th ed.). Cambridge, UK: Royal Society of Chemistry. 2013. ISBN 1849736707. OCLC 824530529.CS1 maint: others (link)
- Sharma, I.; Dhaliwal, L.; Saha, S.; Sangwan, S.; Dhawan, V. (2010). "Role of 8-iso-prostaglandin F2alpha and 25-hydroxycholesterol in the pathophysiology of endometriosis". Fertility and Sterility. 94 (1): 63–70. doi:10.1016/j.fertnstert.2009.01.141. PMID 19324352.
- Samuelsson, B.; Goldyne, M.; Granström, E.; et al. (1978). "Prostaglandins and thromboxanes". Annual Review of Biochemistry. 47: 997–1029. doi:10.1146/annurev.bi.47.070178.005025.
- Hooper, S.B.; Watkins, W.B.; Thorburn, G.D. (1986). "Oxytocin, oxytocin associated neurophysin, and prostaglandin F2 a concentrations in the uteroovarian vein of pregnant and nonpregnant sheep" (PDF). Endocrinology. 119: 2590–2597. doi:10.1210/endo-119-6-2590.
- "Dinoprost tromethamine Injection Advanced Patient Information". Truvn Health Analytics Inc. 2016. Retrieved November 2, 2017.
- Coulthard, G.; Erb, W.; Aggarwal, V. K. (2012). "Stereocontrolled organocatalytic synthesis of prostaglandin PGF2α in seven steps". Nature. 489 (7415): 278–281. doi:10.1038/nature11411. PMID 22895192.
- Corey, E.J.; Cheng, X.M. (1995). The Logic of Chemical Synthesis. Wiley.
- Fortier, M. A.; Krishnaswamy, K.; Danyod, G.; Boucher-Kovalik, S.; Chapdalaine, P. (August 2008). "A postgenomic integrated view of prostaglandins in reproduction: implications for other body systems". Journal of Physiology and Pharmacology. 59 Suppl 1: 65–89. ISSN 1899-1505. PMID 18802217.