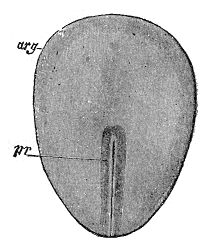
Primitive streak
The primitive streak is a structure that forms in the blastula during the early stages of avian, reptilian and mammalian embryonic development. It forms on the dorsal (back) face of the developing embryo, toward the caudal or posterior end.
| Primitive streak | |
|---|---|
 | |
| Details | |
| Carnegie stage | 6b |
| Days | 15 |
| Identifiers | |
| Latin | linea primitiva |
| MeSH | D054240 |
| Anatomical terminology | |
The presence of the primitive streak will establish bilateral symmetry, determine the site of gastrulation and initiate germ layer formation. To form the streak, reptiles, birds and mammals arrange mesenchymal cells along the prospective midline, establishing the second embryonic axis, as well as the place where cells will ingress and migrate during the process of gastrulation and germ layer formation.[1] The primitive streak extends through this midline and creates the left–right and cranial–caudal body axes,[2] and marks the beginning of gastrulation.[3] This process involves the ingression of mesoderm progenitors and their migration to their ultimate position,[2][4] where they will differentiate into the mesoderm germ layer[1] that, together with endoderm and ectoderm germ layers, will give rise to all the tissues of the adult organism.
Components
Given that the chicken embryo can be easily manipulated, most of our knowledge about the primitive streak comes from avian studies. The marginal zone of a chick embryo contains cells that will contribute to the streak.[4] This region has a defined anterior-to-posterior gradient in its ability to induce the primitive streak, with the posterior end having the highest potential.[5]
The epiblast, a single epithelial layer blastodisc, is the source of all embryonic material in amniotes[1] and some of its cells will give rise to the primitive streak.[4] All cells in the epiblast can respond to signals from the marginal zone,[1] but once a given region is induced by these signals and undergoes streak formation, the remaining cells in the epiblast are no longer responsive to these inductive signals and prevent the formation of another streak.[5]
Underlying the epiblast is the hypoblast, where the extra-embryonic tissue originates.[4] In the chick, the absence of the hypoblast results in multiple streaks,[6] suggesting that its presence is important for regulating the formation of a single primitive streak. In mice, this structure is known as the Anterior Visceral Endoderm (AVE).[6]
Cellular movements
The formation of the primitive streak in the blastocyst involves the coordinated movement and re-arrangement of cells in the epiblast. Even before the streak is visible, epiblast cells have started to move.[7] Two counter-rotating flows of cells meet at the posterior end, where the streak forms.[7] There is little movement in the center of these flows, while the greatest movement is observed at the periphery of the vortices.[3] The Polonaise Movement is key for the formation of the primitive streak. Cells overlaying Koller’s Sickle in the posterior end of the chick embryo move towards the midline, meet and change direction towards the center of the epiblast. Cells from the lateral posterior marginal zone replace those cells that left Koller’s Sickle by meeting at the center of this region, changing direction and extending anteriorly.[4][8] As these cells move and concentrate at the posterior end of the embryo, the streak undergoes a single- to multi-layered epithelial sheet transition that makes it a macroscopically visible structure.[4] Several mechanisms, including oriented cell division, cell-cell intercalation and chemotactic cell movement,[4] have been proposed to explain the nature of the cellular movements required to form the primitive streak.
Formation
The formation of the primitive streak relies on a complex network of signaling pathways that work together to ensure that this process is highly regulated. Activation of various secreted factors (Vg1, Nodal, Wnt8C, FGF8 and Chordin) and transcription factors (Brachyury and Goosecoid) adjacent to the site of streak formation is required for this process.[9][10][11][12][13] In addition, structures such as the hypoblast also play an important role in the regulation of streak formation. Removal of the hypoblast in the chick results in correctly patterned ectopic streaks, suggesting that the hypoblast serves to inhibit formation of the primitive streak.[13]
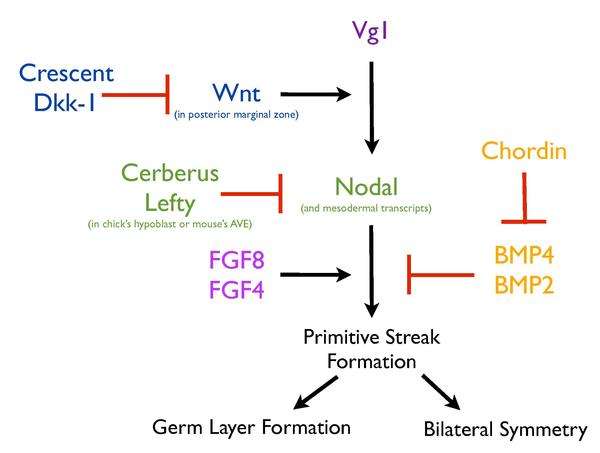
Vg1 and Wnt signaling
Similarly, Vg1 (a TGFB family member) misexpression and grafts of the posterior marginal zone[5] in chicks can also induce ectopic streaks, but only within the marginal zone of the embryo,[11] indicating a specific characteristic of this region in its ability to induce streak formation. Several lines of evidence point to Wnt expression as the determinant of this ability. Deletion of Wnt3 in mouse embryos results in the absence of a streak formation, similarly to the phenotype of B-catenin mutant embryos.[14] In addition, mutating the intracellular negative regulator of Wnt signaling, Axin,[15] and misexpression of the chick cWnt8C[16] produces multiple streaks in mouse embryos. Localization of Wnt and components of its pathway, Lef1 and B-catenin, further supports streak-inducing role in the marginal zone.[11] Furthermore, it is expressed as a gradient decreasing from posterior to anterior,[11][12] corresponding to the streak-inducing ability of the marginal zone. Misexpression of Vg1 or Wnt1 alone failed to induce an ectopic streak in the chick, but together their misexpression resulted in ectopic streak formation, confirming that the streak-inducing ability of the posterior marginal zone could be attributed to Wnt signaling[11] and that Vg1 and Wnt must cooperate to induce this process. Misexpression of Vg1 along with Wnt antagonists, Crescent or Dkk-1, prevents the formation of ectopic streaks,[11] demonstrating the importance of Wnt activity in the formation of Vg1-induced ectopic streaks and hence its implication in normal primitive streak formation.
Hypoblast
Any given slice from the blastoderm is able to generate a complete axis[17] until the time of gastrulation and primitive streak formation.[13] This ability to generate a streak from the pre-streak stage chick embryo[18] indicates that there must be a mechanism to ensure that only a single streak forms. The hypoblast secretes an antagonist of Nodal that prevents ectopic streak formation in the chick.[13]
Nodal signaling
Nodal, a known mesodermal inducer of the TGFB superfamily,[18] has been implicated in streak formation. Mouse embryos mutant for Nodal fail to gastrulate and lack most mesoderm,[19] but more than playing a role in mesoderm induction, Nodal regulates the induction and/or maintenance of the primitive streak.[19] In the presence of hypoblast, Nodal is unable to induce ectopic streaks in the chick embryo, while its removal, induces expression of Nodal, Chordin and Brachyury,[13] suggesting that the hypoblast must have a certain inhibitory effect on Nodal signaling. Indeed, the multifunctional antagonist of Nodal, Wnt and BMP signaling, Cerberus (produced in the hypoblast) and Cerberus-Short (which inhibits only Nodal), through its effect on Nodal signaling, inhibits streak formation.[13] Eventually, the hypoblast gets displaced anteriorly by the moving endoblast, allowing streak formation at the posterior end. At the anterior end, the presence of the hypoblast and the antagonists it secretes, such as Cerberus, inhibit the expression of Nodal and hence restrict streak formation to the posterior end only.[13] Similarly to the hypoblast in chick, the AVE in the mouse secretes two antagonists of Nodal signaling, Cerberus-like, Cerl, and Lefty1.[13][20] In mouse, Cer-/-; Lefty1-/- double mutants develop multiple streaks[6] as indicated by ectopic expression of Brachyury and can be partially rescued by the removal of one copy of the Nodal gene.[6] In the mouse, the AVE restricts streak formation through the redundant functions of Cer1 and Lefty1, which negatively regulate Nodal signaling.[6] The role of the mouse’s AVE in ensuring the formation of a single primitive streak is evolutionarily conserved in the hypoblast of the chick.[6][13]
FGF signaling
Another important pathway in modulating formation of the primitive streak is FGF, which is thought to work together with Nodal to regulate this process.[18] Inhibition of FGF signaling through expression of a dominant negative receptor, using a FGF receptor inhibitor (SU5402) or depletion of FGF ligands, inhibit mesoderm formation[3] and this in turn, inhibits streak formation.[4] Furthermore, ectopic streak formation induced by Vg1 required FGF signaling.[18]
BMP signaling
Finally, BMP signaling is also important for regulating the process of streak formation in the chick embryo. The site of streak formation is characterized by low BMP signals, while the rest of the epiblast displays high levels of BMP activation.[21] In addition, misexpression of either BMP4 or BMP7 prevents streak formation, while the BMP inhibitor Chordin induces ectopic streak formation in the chick,[22] suggesting that streak formation is likely to require BMP inhibition.
Ethical implications
The primitive streak is an important concept in bioethics, where some experts have argued that experimentation with human embryos is permissible, but only before the primitive streak develops, generally around the fourteenth day of existence. The development of the primitive streak is taken, by such bioethicists, to signify the creation of a unique, human being.[23] In some countries, it is illegal to develop a human embryo for more than 14 days outside a woman's body.[24]
Additional images
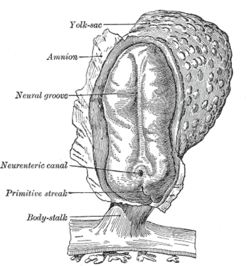 Human embryo—length, 2 mm. Dorsal view, with the amnion laid open. X 30.
Human embryo—length, 2 mm. Dorsal view, with the amnion laid open. X 30.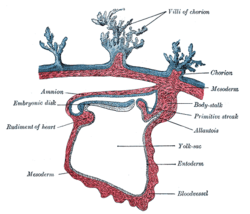 Lateral section through the mammalian blastodisc.
Lateral section through the mammalian blastodisc.
References
- Mikawa T, Poh AM, Kelly KA, Ishii Y, Reese DE (2004). "Induction and patterning of the primitive streak, an organizing center of gastrulation in the amniote". Dev Dyn. 229 (3): 422–32. doi:10.1002/dvdy.10458. PMID 14991697.
- Downs KM (2009). "The enigmatic primitive streak: prevailing notions and challenges concerning the body axis of mammals". BioEssays. 31 (8): 892–902. doi:10.1002/bies.200900038. PMC 2949267. PMID 19609969.
- Chuai M, Zeng W, Yang X, Boychenko V, Glazier JA, Weijer CJ (2006). "Cell movement during chick primitive streak formation". Dev. Biol. 296 (1): 137–49. doi:10.1016/j.ydbio.2006.04.451. PMC 2556955. PMID 16725136.
- Chuai M, Weijer CJ (2008). "The mechanisms underlying primitive streak formation in the chick embryo". Curr Top Dev Biol. Current Topics in Developmental Biology. 81: 135–56. doi:10.1016/S0070-2153(07)81004-0. ISBN 978-0-12-374253-7. PMID 18023726.
- Khaner O, Eyal-Giladi H (1989). "The chick's marginal zone and primitive streak formation. I. Coordinative effect of induction and inhibition". Dev. Biol. 134 (1): 206–14. doi:10.1016/0012-1606(89)90090-0. PMID 2731648.
- Perea-Gomez A, Vella FD, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H, Behringer RR, Ang SL (2002). "Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks". Dev Cell. 3 (5): 745–56. doi:10.1016/S1534-5807(02)00321-0. PMID 12431380.
- Cui C, Yang X, Chuai M, Glazier JA, Weijer CJ (2005). "Analysis of tissue flow patterns during primitive streak formation in the chick embryo". Dev. Biol. 284 (1): 37–47. doi:10.1016/j.ydbio.2005.04.021. PMID 15950214.
- Hatada Y, Stern CD (1994). "A fate map of the epiblast of the early chick embryo". Development. 120 (10): 2879–89. PMID 7607078.
- Shah SB, Skromne I, Hume CR, Kessler DS, Lee KJ, Stern CD, Dodd J (1997). "Misexpression of chick Vg1 in the marginal zone induces primitive streak formation". Development. 124 (24): 5127–38. PMID 9362470.
- Bachvarova RF, Skromne I, Stern CD (1998). "Induction of primitive streak and Hensen's node by the posterior marginal zone in the early chick embryo". Development. 125 (17): 3521–34. PMID 9693154.
- Skromne I, Stern CD (2001). "Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo". Development. 128 (15): 2915–27. PMID 11532915.
- Skromne I, Stern CD (2002). "A hierarchy of gene expression accompanying induction of the primitive streak by Vg1 in the chick embryo". Mech. Dev. 114 (1–2): 115–8. doi:10.1016/S0925-4773(02)00034-5. PMID 12175495.
- Bertocchini F, Stern CD (2002). "The hypoblast of the chick embryo positions the primitive streak by antagonizing nodal signaling". Dev Cell. 3 (5): 735–44. doi:10.1016/S1534-5807(02)00318-0. PMID 12431379.
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A (1999). "Requirement for Wnt3 in vertebrate axis formation". Nat. Genet. 22 (4): 361–5. doi:10.1038/11932. PMID 10431240.
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F (1997). "The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation". Cell. 90 (1): 181–92. doi:10.1016/S0092-8674(00)80324-4. PMID 9230313.
- Pöpperl H, Schmidt C, Wilson V, Hume CR, Dodd J, Krumlauf R, Beddington RS (1997). "Misexpression of Cwnt8C in the mouse induces an ectopic embryonic axis and causes a truncation of the anterior neuroectoderm". Development. 124 (15): 2997–3005. PMID 9247341.
- SPRATT NT Jr; HAAS H. (1960). "Integrative mechanisms in development of the early chick blastoderm. I. REgulative potentiality of separated parts". J Exp Zool. 145 (2): 97–137. doi:10.1002/jez.1401450202.
- Bertocchini F, Skromne I, Wolpert L, Stern CD (2004). "Determination of embryonic polarity in a regulative system: evidence for endogenous inhibitors acting sequentially during primitive streak formation in the chick embryo". Development. 131 (14): 3381–90. doi:10.1242/dev.01178. PMID 15226255.
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ (1994). "A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse". Development. 120 (7): 1919–28. PMID 7924997.
- C Perea-Gomez A, Rhinn M, Ang SL (2001). "Role of the anterior visceral endoderm in restricting posterior signals in the mouse embryo". Int J Dev Biol. 45 (1): 311–20. PMID 11291861.
- Faure S, de Santa Barbara P, Roberts DJ, Whitman M (2002). "Endogenous patterns of BMP signaling during early chick development". Dev. Biol. 244 (1): 44–65. doi:10.1006/dbio.2002.0579. PMID 11900458.
- Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stern CD (1998). "Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo". Development. 125 (3): 507–19. PMID 9425145.
- "The President's Council on Bioethics, Human Cloning and Human Dignity: An Ethical Inquiry. Chapter 6". July 2002.
- "Prohibition of Human Cloning for Reproduction Act 2002". Government of Australia Department of Health and Ageing. 22 Dec 2008.