Pretomanid
Pretomanid is an antibiotic used for the treatment of multi-drug-resistant tuberculosis affecting the lungs.[1] It is generally used together with bedaquiline and linezolid.[1] It is taken by mouth.[1]
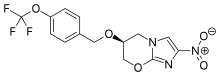 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H12F3N3O5 |
| Molar mass | 359.261 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The most common side effects include nerve damage, acne, vomiting, headache, low blood sugar, diarrhea, and liver inflammation.[1] It is in the nitroimidazole class of medications.[2]
Pretomanid was approved for medical use in the United States in 2019.[1] Pretomanid was developed by TB Alliance.[3][4][5]
History
Pretomanid is the generic, nonproprietary name for the novel anti-bacterial drug compound formerly called PA-824.[6] Pretomanid is referred to as "Pa" in regimen abbreviations, such as BPaL. The "preto" prefix of the compound's name honors Pretoria, South Africa, the home of a TB Alliance clinical development office where much of the drug's development took place. The "manid" suffix is used to group compounds with similar chemical structures. This class of drug is variously referred to as nitroimidazoles, nitroimidazooxazines or nitroimidazopyrans. Development of this compound was initiated because of the urgent need for new antibacterial drugs effective against resistant strains of tuberculosis. Also, current anti-TB drugs are mainly effective against replicating and metabolically active bacteria, creating a need for drugs effective against persisting or latent bacterial infections as often occur in patients with tuberculosis.[7]
Discovery and pre-clinical development
Pretomanid was first identified in a series of 100 nitroimidazopyran derivatives synthesized and tested for antitubercular activity. Importantly, pretomanid has activity against static M. tuberculosis isolates that survive under anaerobic conditions, with bactericidal activity comparable to that of the existing drug metronidazole. Pretomanid requires metabolic activation by Mycobacterium for antibacterial activity. Pretomanid was not the most potent compound in the series against cultures of M. tuberculosis, but it was the most active in infected mice after oral administration. Oral pretomanid was active against tuberculosis in mice and guinea pigs at safely tolerated dosages for up to 28 days.[7]
Limited FDA approval
FDA approved pretomanid only in combination with bedaquiline and linezolid for treatment of a limited and specific population of adult patients with extensively drug resistant, treatment-intolerant or nonresponsive multidrug resistant pulmonary tuberculosis.[1] Pretomanid was approved under the Limited Population Pathway (LPAD pathway) for antibacterial and antifungal drugs.[1] The LPAD Pathway was established by Congress under the 21st Century Cures Act to expedite development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need. Pretomanid is only the third tuberculosis drug to receive FDA approval in more than 40 years.[1][5]
The FDA granted Pretomanid priority review and orphan drug designation.[1] The FDA granted The Global Alliance for TB Drug Development (TB Alliance) the approval of Pretomanid and a Tropical Disease Priority Review Voucher.[1]
See also
- Delamanid, a related drug
References
- "FDA approves new drug for treatment-resistant forms of tuberculosis that affects the lungs". FDA. 14 August 2019. Retrieved 28 August 2019.
- "Compounds | TB Alliance". www.tballiance.org. Retrieved 18 April 2019.
- "TB Medicine Pretomanid Enters Regulatory Review Process in the United States". TB Alliance. Retrieved 18 April 2019.
- "FDA approves new drug for treatment-resistant forms of tuberculosis that affects the lungs". U.S. Food and Drug Administration (FDA) (Press release). 14 August 2019. Retrieved 17 November 2019.

- Abutaleb Y (14 August 2019). "New antibiotic approved for drug-resistant tuberculosis". The Washington Post.
- "PA-824 has a New Generic Name: Pretomanid". TB Alliance. Retrieved 18 April 2019.
- Lenaerts AJ, Gruppo V, Marietta KS, Johnson CM, Driscoll DK, Tompkins NM, Rose JD, Reynolds RC, Orme IM (June 2005). "Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models". Antimicrobial Agents and Chemotherapy. 49 (6): 2294–301. doi:10.1128/AAC.49.6.2294-2301.2005. PMC 1140539. PMID 15917524.
External links
- "Pretomanid". Drug Information Portal. U.S. National Library of Medicine.