Phototoxicity
Phototoxicity, also called photoirritation, is a chemically induced skin irritation, requiring light, that does not involve the immune system.[1] It is a type of photosensitivity.[1][2]
| Phototoxicity | |
|---|---|
| Other names | Photoirritation |
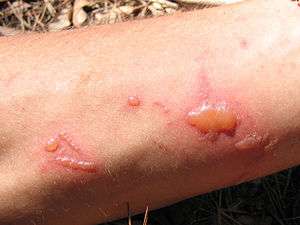 | |
| Effect of the common rue on skin in hot weather. | |
| Specialty | Dermatology |
The skin response resembles an exaggerated sunburn. The involved chemical may enter into the skin by topical administration or it may reach the skin via systemic circulation following ingestion or parenteral administration. The chemical needs to be "photoactive," which means that when it absorbs light, the absorbed energy produces molecular changes that cause toxicity. Many synthetic compounds, including drug substances like tetracyclines or fluoroquinolones, are known to cause these effects. Surface contact with some such chemicals causes photodermatitis; many plants cause phytophotodermatitis. Light-induced toxicity is a common phenomenon in humans; however, it also occurs in other animals.
Scientific background
A phototoxic substance is a chemical compound which becomes toxic when exposed to light.
- Some medicines: tetracycline antibiotics, sulfonamides, amiodarone, quinolones, psoralen
- Many cold pressed citrus essential oils such as bergamot oil[3]
- Some plant juices: parsley, lime, and Heracleum mantegazzianum
Phototoxicity is a quantum chemical phenomenon. Phototoxins are molecules with a conjugated system, often an aromatic system. They have a low-lying excited state that can be reached by excitation with visible light photons. This state can undergo intersystem crossing with neighboring molecules in tissue, converting them to toxic free radicals. These rapidly attack nearby molecules, killing cells. A typical radical is singlet oxygen, produced from regular triplet oxygen. Because free radicals are highly reactive, the damage is limited to the body part illuminated.
Photosafety evaluation
Physico-chemical properties
In vitro test systems
3T3 Neutral Red Phototoxicity Test – An in vitro toxicological assessment test used to determine the cytotoxic and photo(cyto)toxicity effect of a test article to murine fibroblasts in the presence or absence of UVA light.
"The 3T3 Neutral Red Uptake Phototoxicity Assay (3T3 NRU PT) can be utilized to identify the phototoxic effect of a test substance induced by the combination of test substance and light and is based on the comparison of the cytotoxic effect of a test substance when tested after the exposure and in the absence of exposure to a non-cytotoxic dose of UVA/vis light. Cytotoxicity is expressed as a concentration-dependent reduction of the uptake of the vital dye - Neutral Red.
Substances that are phototoxic in vivo after systemic application and distribution to the skin, as well as compounds that could act as phototoxicants after topical application to the skin can be identified by the test. The reliability and relevance of the 3T3 NRU PT have been evaluated and has been shown to be predictive when compared with acute phototoxicity effects in vivo in animals and humans." Taken with permission from
During drug development
Several health authorities have issued related guidance documents, which need to be considered for drug development:
- ICH (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use)
- EMA (European Medicines Agency)
- FDA (U.S. Food and Drug Administration)
- MHLW/PMDA (Japanese Ministry of Health, Labour and Welfare / Pharmaceuticals and Medical Devices Agency)
See also
References
- Anderson, D.M.; Keith, J.; Novac, P.; Elliott, M.A., eds. (1994). Dorland's Illustrated Medical Dictionary (28th ed.). W. B. Saunders Company. ISBN 0721655777.
- JH Epstein (1999). "Phototoxicity and photoallergy". Seminars in Cutaneous Medicine and Surgery. 18 (4): 274–284. PMID 10604793.
- "Bergamot oil". Drugs.com. 2018. Retrieved 5 December 2018.
- "Multidisciplinary Guidelines". ICH. Retrieved 2013-08-06.
- "Safety Guidelines". ICH. Retrieved 2013-08-06.
- "European Medicines Agency - Non-clinical: Toxicology". Ema.europa.eu. 2010-06-24. Retrieved 2013-08-06.