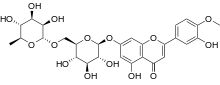
Diosmin
Diosmin (diosmetin 7-O-rutinoside), a flavone glycoside of diosmetin, is manufactured from citrus fruit peels as a non-prescription dietary supplement used to aid treatment of hemorrhoids or chronic venous diseases, mainly of the legs.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | oral |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.537 |
| Chemical and physical data | |
| Formula | C28H32O15 |
| Molar mass | 608.545 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Uses
Diosmin is a dietary supplement used to aid treatment of hemorrhoids and venous diseases, i.e., chronic venous insufficiency including spider and varicose veins, leg swelling (edema), stasis dermatitis and venous ulcers.[1] The mechanism of action of Diosmin and other phlebotonics is undefined,[1] and clinical evidence of benefit is limited.[1] Diosmin is not recommended for treating the rectal mucosa, skin irritations, or wounds, and should not be used to treat dermatitis, eczema, or urticaria.[1] Diosmin is not recommended for use in children or women during pregnancy.[1]
Phlebotonics
Diosmin is included among a small class of agents called "phlebotonics" having heterogeneous composition and consisting partly of citrus peel extracts (flavonoids, such as hesperidin) and synthetic compounds, which are used to treat chronic venous insufficiency or hemorrhoids.[1] A 2016 Cochrane review found only moderate-quality evidence that diosmin or other phlebotonics improved leg and ankle swelling and lower leg pain,[2] and a 2012 review indicated low-quality evidence for treating hemorrhoids.[1][3]
In 2017, the American Working Group in Chronic Venous Disease recommended use of micronized purified flavonoid fraction (diosmiplex) as a medical food for chronic venous disease symptoms and venous ulcers, having "beneficial outcomes without serious adverse events", alone or combined with compression therapy, concurring with the previous guidance of the International European Society for Vascular Surgery.[4] The German Dermatological Society indicated that Diosmin may be used with other treatments for symptoms of chronic venous diseases.[5]
The American Society of Colon and Rectal Surgeons mentions phlebotonics as a possible treatment for symptoms of hemorrhoid grades I to II, as there is only moderate-quality evidence of effectiveness with "expectations of minimal harm", while having no evidence of long-term benefit.[3][6] French,[7][8] Indian,[9] Portuguese,[10] and Italian[11] professional societies of coloproctology issued similar recommendations regarding phlebotonics for hemorrhoids.
Adverse effects
In some 10% of users, diosmin causes mild gastrointestinal disorders or skin irritations (hives, itching), stomach pain, nausea, heart arrhythmias, or anemia.[1] Preliminary research indicates no evidence of toxicity.[1] The US Food and Drug Administration (FDA) concluded in 2001 that there was inadequate evidence on which to base an expectation of safety.[12] As of 2013, the FDA did not revise this position.[13]
Regulatory status
Diosmin is distributed in the U.S. as a dietary supplement called Daflon.[1] Diosmin is not approved as a prescription drug in the United States or Europe.[1] Phlebotonics are not approved in Germany,[5] and are restricted in Spain only for the treatment of chronic venous diseases.[14]
Diosmiplex
Micronized purified flavonoid fraction (generic name diosmiplex, commercial name Vasculera)[4] is a 2nd generation diosmin classified as a medical food by the FDA in the United States for the potential treatment of chronic venous insufficiency.[15][16] Diosmiplex is the only phlebotonic, as of 2017, sold as a medical food product purported for the management of chronic venous insufficiency.[4] Diosmiplex is recommended by the American Working Group in Chronic Venous Disease as a medical therapy, alone or combined with compressive therapy, for chronic venous disease symptoms and venous ulcers, having "beneficial outcomes without serious adverse events", concurring with the previous guidance of the International European Society for Vascular Surgery.[4]
See also
- Daflon
- Venalex
References
- "Diosmin". Drugs.com. 1 January 2019. Retrieved 7 November 2019.
- Martinez-Zapata, Maria José; Vernooij, Robin WM; Uriona Tuma, Sonia Maria; Stein, Airton T; Moreno, Rosa M; Vargas, Emilio; Capellà, Dolors; Bonfill Cosp, Xavier (6 April 2016). "Phlebotonics for venous insufficiency". Cochrane Database of Systematic Reviews. 4: CD003229. doi:10.1002/14651858.CD003229.pub3. PMID 27048768.
- Perera, Nirmal; Liolitsa, Danae; Iype, Satheesh; Croxford, Anna; Yassin, Muhammed; Lang, Peter; Ukaegbu, Obioha; van Issum, Christopher (15 August 2012). "Phlebotonics for haemorrhoids". Cochrane Database of Systematic Reviews (8): CD004322. doi:10.1002/14651858.CD004322.pub3. PMID 22895941.
- Bush, R; Comerota, A; Meissner, M; Raffetto, JD; Hahn, SR; Freeman, K (April 2017). "Recommendations for the medical management of chronic venous disease: The role of Micronized Purified Flavanoid Fraction (MPFF): Recommendations from the Working Group in Chronic Venous Disease (CVD) 2016". Phlebology (Professional society guidelines). 32 (1_suppl): 3–19. doi:10.1177/0268355517692221. PMID 28211296.
Only recently has a standardized formulation MPFF been available in the United States (Vasculera—Primus Pharmaceuticals, Inc.).
- Stücker, M; Debus, ES; Hoffmann, J; Jünger, M; Kröger, K; Mumme, A; Ramelet, AA; Rabe, E (June 2016). "Consensus statement on the symptom-based treatment of chronic venous diseases". Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology (Professional society guidelines). 14 (6): 575–83. doi:10.1111/ddg.13006. PMID 27240062.
- Davis, BR; Lee-Kong, SA; Migaly, J; Feingold, DL; Steele, SR (March 2018). "The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Hemorrhoids". Diseases of the Colon and Rectum (Professional society guidelines). 61 (3): 284–292. doi:10.1097/DCR.0000000000001030. PMID 29420423.
- Higuero, T; Abramowitz, L; Castinel, A; Fathallah, N; Hemery, P; Laclotte Duhoux, C; Pigot, F; Pillant-Le Moult, H; Senéjoux, A; Siproudhis, L; Staumont, G; Suduca, JM; Vinson-Bonnet, B (June 2016). "Guidelines for the treatment of hemorrhoids (short report)". Journal of Visceral Surgery (Professional society guidelines). 153 (3): 213–8. doi:10.1016/j.jviscsurg.2016.03.004. PMID 27209079.
- Higuero, T (December 2017). "Treatment of hemorroïdal disease: new french guidelines". La Revue du praticien (in French). 67 (10): 1059–1062. PMID 30512599.
- Agarwal, Niranjan; Singh, Kumkum; Sheikh, Parvez; Mittal, Kushal; Mathai, Varughese; Kumar, Ashok (9 January 2017). "Executive Summary - The Association of Colon & Rectal Surgeons of India (ACRSI) Practice Guidelines for the Management of Haemorrhoids—2016". Indian Journal of Surgery (Professional society guidelines). 79 (1): 58–61. doi:10.1007/s12262-016-1578-7. PMC 5346092. PMID 28331268.
- Salgueiro, Paulo; Caetano, Ana Célia; Oliveira, Ana Maria; Rosa, Bruno; Mascarenhas-Saraiva, Miguel; Ministro, Paula; Amaro, Pedro; Godinho, Rogério; Coelho, Rosa; Gaio, Rúben; Fernandes, Samuel; Fernandes, Vítor; Castro-Poças, Fernando (5 September 2019). "Portuguese Society of Gastroenterology Consensus on the Diagnosis and Management of Hemorrhoidal Disease". GE - Portuguese Journal of Gastroenterology (Professional society guidelines): 1–13. doi:10.1159/000502260.
- Trompetto, M.; Clerico, G.; Cocorullo, G. F.; Giordano, P.; Marino, F.; Martellucci, J.; Milito, G.; Mistrangelo, M.; Ratto, C. (24 September 2015). "Evaluation and management of hemorrhoids: Italian society of colorectal surgery (SICCR) consensus statement". Techniques in Coloproctology. 19 (10): 567–575. doi:10.1007/s10151-015-1371-9. hdl:10447/208054. PMID 26403234.
- New Dietary Ingredients in Dietary Supplements, U. S. Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Nutritional Products, Labeling, and Dietary Supplements February 2001 (Updated September 10, 2001) , Memorandum
- Garg, Nitin; Gloviczki, Peter (2013). "55 - Chronic Venous Insufficiency". Vascular Medicine: A Companion to Braunwald's Heart Disease (Second Edition). Elsevier Health Sciences. pp. 652–666. ISBN 9781437729306.
- "Consolidated List of Products - Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or Not Approved by Governments, Twelfth Issue - Pharmaceuticals. United Nations - New York, 2005". apps.who.int. 2005. Retrieved 7 November 2019.
- "DailyMed - Vasculera- diosmiplex tablet". dailymed.nlm.nih.gov. National Institutes of Health. 26 April 2012. Retrieved 8 November 2019.
- "Vasculera". Drugs.com. 3 December 2018. Retrieved 7 November 2019.