Perflubron
Perflubron (INN/USAN, or perfluorooctyl bromide; brand name Imagent) is a contrast medium for magnetic resonance imaging, computer tomography and sonography.[1] It was approved for this use in the United States by the Food and Drug Administration in 1993.[2]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.391 |
| Chemical and physical data | |
| Formula | C8BrF17 |
| Molar mass | 498.96 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.93 g/cm3 |
| Melting point | 6 °C (43 °F) |
| Boiling point | 142 °C (288 °F) |
SMILES
| |
InChI
| |
Experimental research
Perflubron has also been tested experimentally for use in liquid breathing in premature infants with respiratory distress.[3][4]
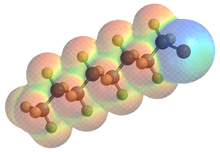
This molecular model of Perflubron is color-coded by electron density to illustrate the positive polarisation of the large bromine atom by the inductively electron-withdrawing perfluorooctyl chain.
References
- Mattrey, R. F. (1989). "Perfluorooctylbromide: A new contrast agent for CT, sonography, and MR imaging". AJR. American Journal of Roentgenology. 152 (2): 247–252. doi:10.2214/ajr.152.2.247. PMID 2643258.
- FDA Approved Drug Products
- Wolfson, M. R.; Kechner, N. E.; Roache, R. F.; Dechadarevian, J. P.; Friss, H. E.; Rubenstein, S. D.; Shaffer, T. H. (1998). "Perfluorochemical rescue after surfactant treatment: Effect of perflubron dose and ventilatory frequency". Journal of Applied Physiology. 84 (2): 624–640. doi:10.1152/jappl.1998.84.2.624. PMID 9475875.
- Leach, C. L.; Greenspan, J. S.; Rubenstein, S. D.; Shaffer, T. H.; Wolfson, M. R.; Jackson, J. C.; Delemos, R.; Fuhrman, B. P. (1996). "Partial Liquid Ventilation with Perflubron in Premature Infants with Severe Respiratory Distress Syndrome". New England Journal of Medicine. 335 (11): 761–767. doi:10.1056/NEJM199609123351101. PMID 8778584.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.