Pegdinetanib
Pegdinetanib (USAN; planned trade name Angiocept) is an investigational anti-cancer drug that acts as a selective antagonist of vascular endothelial growth factor receptor 2 (VEGFR-2), hindering vascularization of tumors. It is a genetically engineered peptide derivative based on the monobody technology, and is being developed by Adnexus.[1][2]
 | |
| Clinical data | |
|---|---|
| Trade names | Angiocept |
| Other names | CT-322; BMS-844203 |
| Routes of administration | Intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| KEGG | |
| Chemical and physical data | |
| Formula | C468H729N125O139S (un-PEGylated peptide) |
| Molar mass | 10362.78 g·mol−1 |
The drug has entered Phase II clinical trials investigating the treatment of glioblastoma in October 2007.[3][4] As of August 2012, it is also in Phase II trials for the treatment of non-small cell lung cancer[5] and colorectal cancer.[6]
Chemical structure
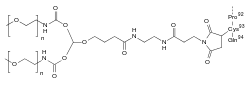
Pegdinetanib is a peptide consisting of 94 amino acids, with cysteine number 93 carrying a doubly methoxy-PEGylated maleimide derivative with a molecular mass of 40 kDa.[7]
References
- Tolcher, A. W.; Sweeney, C. J.; Papadopoulos, K.; Patnaik, A.; Chiorean, E. G.; Mita, A. C.; Sankhala, K.; Furfine, E.; Gokemeijer, J.; Iacono, L.; Eaton, C.; Silver, B. A.; Mita, M. (2011). "Phase I and Pharmacokinetic Study of CT-322 (BMS-844203), a Targeted Adnectin Inhibitor of VEGFR-2 Based on a Domain of Human Fibronectin". Clinical Cancer Research. 17 (2): 363–371. doi:10.1158/1078-0432.CCR-10-1411. PMID 21224368.
- Mamluk, R.; Carvajal, I. M.; Morse, B. A.; Wong, H.; Abramowitz, J.; Aslanian, S.; Lim, A. C.; Gokemeijer, J.; Storek, M. J.; Lee, J.; Gosselin, M.; Wright, M. C.; Camphausen, R. T.; Wang, J.; Chen, Y.; Miller, K.; Sanders, K.; Short, S.; Sperinde, J.; Prasad, G.; Williams, S.; Kerbel, R.; Ebos, J.; Mutsaers, A.; Mendlein, J. D.; Harris, A. S.; Furfine, E. S. (2010). "Anti-tumor effect of CT-322 as an adnectin inhibitor of vascular endothelial growth factor receptor-2". MAbs. 2 (2): 199–208. doi:10.4161/mabs.2.2.11304. PMC 2840239. PMID 20190562.
- Clinical trial number NCT00562419 for "CT-322 in Treating Patients With Recurrent Glioblastoma Multiforme and Combination Therapy With Irinotecan" at ClinicalTrials.gov
- Bloom, L.; Calabro, V. (2009). "FN3: A new protein scaffold reaches the clinic". Drug Discovery Today. 14 (19–20): 949–955. doi:10.1016/j.drudis.2009.06.007. PMID 19576999.
- Clinical trial number NCT00850577 for "Ph II of a Novel Anti-angiogenic Agent in Combination With Chemotherapy for the Treatment of Non-Small Cell Lung Cancer" at ClinicalTrials.gov
- Clinical trial number NCT00851045 for "Ph II Trial of a Novel Anti-angiogenic Agent in Combination With Chemotherapy for the Second-line Treatment of Metastatic Colorectal Cancer" at ClinicalTrials.gov
- Statement On A Nonproprietary Name Adopted By The USAN Council: Pegdinetanib