Nutrition facts label
The nutrition facts label (also known as the nutrition information panel, and other slight variations) is a label required on most packaged food in many countries, showing what nutrients (to limit and get enough of) are in the food. Labels are usually based on official nutritional rating systems. Most countries also release overall nutrition guides for general educational purposes. In some cases, the guides are based on different dietary targets for various nutrients than the labels on specific foods.
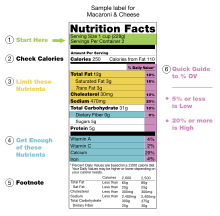
Nutrition facts labels are one of many types of food labels required by regulation or applied by manufacturers.
Australia and New Zealand
Australia and New Zealand use a nutritional information panel of the following format:
|
Servings per package: Serving size: g | ||
| Quantity per Serving | Quantity per 100 g | |
|---|---|---|
| Energy | 0 | kJ (Cal) |
| Protein | 0 | g |
| Fat, total | 0 | g |
| - saturated | 0 | g |
| Carbohydrate | g | g |
| - sugars | g | g |
| Sodium | mg | mg |
Other items are included as appropriate, and the units may be varied as appropriate (e.g. substituting ml for g, or mmol for mg in the 'Sodium' row).[2] In April 2013 the New Zealand government introduced rules around common claims made on food packaging, such as 'low in fat'.[3]
Canada

In Canada, a standardized "Nutrition Facts" label was introduced as part of regulations passed in 2003, and became mandatory for most prepackaged food products on December 12, 2005. (Smaller businesses were given until December 12, 2007 to make the information available.)[4] In accordance with food packaging laws in the country, all information, including the nutrition label, must be written in both English and French, the country's two official languages.[5]
Canadian regulation tightly controls the manner in which the nutrition fact table (NFT) data are laid out. There is a variety of possible formats for use on a given food package. A hierarchy is used to select among the formats (28 main formats, and 2-7 sub formats for each). This results in standard (vertical) formats being considered for use before horizontal and linear formats. The selection hierarchy also allows the NFT to occupy no more than 15% of the physical package's available display area (ADS), but never to be smaller than a format that would be less than 15% of ADS. In practice, determining the ADS of a package, and selecting the appropriate NFT format, can be a detailed calculation.
China
In 2011 the Chinese Ministry of Health released the National Food Safety Standard for Nutrition Labeling of Prepackaged Foods (GB 28050-2011). The core nutrients that must be on a label are: protein, fat, carbohydrate and sodium. Energy is noted in kJ. And all values must be per 100g/100ml.[6][7]
European Union
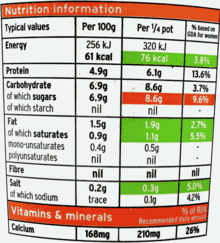
It was regulated by the Commission Directive 2008/100/EC of 28 October 2008 amending Council Directive 90/496/EEC on nutrition labelling for foodstuffs as regards recommended daily allowances, energy conversion factors and definitions.[8] A new regulation is now in force (Regulation 1169/2011).[9] Nutritional labelling becomes mandatory for most pre-packaged foods as from December 2016.
In the European Union, along the "old" rules (Directive 90/496, amended), the information (usually in panel format) is most often labelled "Nutrition Information" (or equivalent in other EU languages). An example is shown on the right. The panel is optional, but if provided, the prescribed content and format must be followed. It will always give values for a set quantity — 100 g (3.5 oz) or 100 ml (3.5 imp fl oz; 3.4 US fl oz) of the product — and often also for a defined "serving", as an option. First will come the energy values, in both kilocalories and kilojoules.
Then will come a breakdown of constituent elements: usually most or all of protein, carbohydrate, starch, sugar, fat, fibre and sodium. The "fat" figure is likely to be further broken down into saturated and unsaturated fat, while the "carbohydrate" figure is likely to give a subtotal for sugars. With the "new" rules, the mandatory information is: energy, fat, saturates, carbohydrates, sugars, protein and salt, in that particular order, with options to extend this list to: mono-unsaturates, polyunsaturates, polyols, starch, fibre, and vitamins and minerals.[9]
With regards to health claims and nutrition (composition) claims, these are harmonised in the EU through Regulation 1924/2006, amended.[10] In November 2012, the European Commission published two new regulations: Regulation (EC) No. 1047/2012 and Regulation (EC) No.1048/2012. Certain nutrition claim groups as of Regulation (EC) No 1924/2006 had to be changed. Moreover, the health claims associated to barley beta-gluten were amended (e.g. lowering blood cholesterol).[11][12]
Within Regulation 1924, there are legal definitions of terms such as "low fat", "high fibre", "reduced calories".[10]
All health claims have been harmonised in the European Union. They can be used if they have been approved by EFSA. The list of approved and rejected claims is available on a web site.[13]
Provided the full nutrition information is shown on the packet, additional nutritional information and formats (e.g. a traffic light rating system) may be included and this falls outside the scope of regulation.
The United Kingdom regulations are given in Schedules 6 and 7 of the Food Labelling Regulations 1996.[14]
Hong Kong
In Hong Kong nutrition facts labels are regulated by the subsidiary legislation Food and Drugs (Composition and Labelling) (Amendment: Requirements for Nutrition Labelling and Nutrition Claim) Regulation 2008.[15]
India
The Ministry of Health and Family Welfare had, on September 19, 2008, notified the Prevention of Food Adulteration (5th Amendment) Rules, 2008, mandating packaged food manufacturers to declare on their product labels nutritional information and a mark from the F.P.O or Agmark (Companies that are responsible for checking food products) to enable consumers to make informed choices while purchasing.[16] Prior to this amendment, disclosure of nutritional information was largely voluntary though many large manufacturers tended to adopt the international practice.[17]
Mexico
Food products sold in Mexico use the NOM-051-SCFI-1994 "Información nutrimental" product labelling standard, very similar to "Nutrition Facts" in the US. The Official Mexican Standard, or NOM (Norma Oficial Mexicana), was developed by the Mexican Secretary of Commerce and Industrial Promotion (Secretaría de Comercio y Fomento Industrial), now a part of the Secretary of the Economy (SECOFI). It entered into effect on January 24, 1996[18] and defines "General specifications for labelling foods and pre-bottled non-alcoholic beverages."[19]
United States
Description
In the United States, the Nutritional Facts label lists the percentage supplied that is recommended to be met, or to be limited, in one day of human nutrients based on a daily diet of 2,000 calories.
With certain exceptions, such as foods meant for babies, the following Daily Values are used.[20] These are called Reference Daily Intake (RDI) values and were originally based on the highest 1968 Recommended Dietary Allowances (RDA) for each nutrient in order to assure that the needs of all age and sex combinations were met.[21] These are older than the current Recommended Dietary Allowances of the Dietary Reference Intake. For vitamin C, vitamin D, vitamin E, vitamin K, calcium, phosphorus, magnesium, and manganese, the current highest RDAs are up to 50% higher than the older Daily Values used in labeling, whereas for other nutrients the recommended needs have gone down. A side-by-side table of the old and new adult Daily Values is provided at Reference Daily Intake. As of October 2010, the only micronutrients that are required to be included on all labels are vitamin A, vitamin C, calcium, and iron.[22] To determine the nutrient levels in the foods, companies may develop or use databases, and these may be submitted voluntarily to the U.S. Food and Drug Administration for review.[23]
| Nutrient | Daily Value for label (before 2016 update) |
highest RDA of DRI |
unit |
|---|---|---|---|
| Vitamin A | 5,000 | 3,000 | IU |
| Vitamin C | 60 | 90 | mg |
| Thiamin | 1.5 | 1.2 | mg |
| Riboflavin | 1.7 | 1.3 | mg |
| Niacin | 20 | 16 | mg |
| Pantothenic acid | 10 | 5 | mg |
| Vitamin B6 | 2 | 1.7 | mg |
| Folate | 400 | 400 | μg |
| Biotin | 300 | 30 | μg |
| Vitamin B12 | 6 | 2.4 | μg |
| Vitamin D | 400 | 600 | IU |
| Vitamin E | 12 | 15 | mg |
| Vitamin K | 80 | 120 | μg |
| Calcium | 1,000 | 1,300 | mg |
| Iron | 18 | 18 | mg |
| Phosphorus | 1,000 | 1,250 | mg |
| Iodine | 150 | 150 | μg |
| Magnesium | 400 | 420 | mg |
| Zinc | 15 | 11 | mg |
| Selenium | 70 | 55 | μg |
| Copper | 2 | 0.9 | mg |
| Manganese | 2 | 2.3 | mg |
| Chromium | 120 | 35 | μg |
| Molybdenum | 75 | 45 | μg |
| Chloride | 3,400 | 2,300 | mg |
Additionally, there is a requirement for ingredients to be listed in order from highest to lowest quantity, according to their weight.[24]
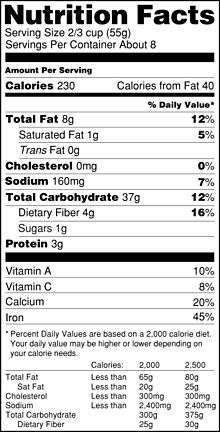

The label was mandated for most food products under the provisions of the 1990 Nutrition Labeling and Education Act (NLEA), per the recommendations of the U.S. Food and Drug Administration.[25] It was one of several controversial actions taken during the tenure of FDA Commissioner Dr. David Kessler. The law required food companies to begin using the new food label on packaged foods beginning May 8, 1994. (Meat and poultry products were not covered by NLEA, though the U.S. Department of Agriculture proposed similar regulations for voluntary labeling of raw meat and poultry.[26]) Foods labeled before that day could use the old label. This appeared on all products in 1995. The old label was titled "Nutrition Information Per Serving" or simply, "Nutrition Information".
The label begins with a standard serving measurement, calories are listed second, and then following is a breakdown of the constituent elements. Always listed are total fat, sodium, carbohydrates and protein; the other nutrients usually shown may be suppressed, if they are zero. Usually all 15 nutrients are shown: calories, calories from fat, fat, saturated fat, trans fat, cholesterol, sodium, carbohydrates, dietary fiber, sugars, protein, vitamin A, vitamin C, calcium, and iron.
Products containing less than 5 g of fat show amounts rounded to the nearest 0.5 g. Amounts less than 0.5 g are rounded to 0 g. For example, if a product contains 0.45 g of trans fat per serving, and the package contains 18 servings, the label would show 0 g of trans fat, even though the product actually contains a total of 8.1 g of trans fat.
In addition to the nutrition label, products may display certain nutrition information or health claims on packaging. These health claims are only allowed by the FDA for "eight diet and health relationships based on proven scientific evidence", including: calcium and osteoporosis, fiber-containing grain products, fruits and vegetables and cancer, fruits, vegetables, and grain products that contain fiber—particularly soluble fiber—and the risk of coronary heart disease, fat and cancer, saturated fat and cholesterol and coronary heart disease, sodium and hypertension, and folate and neural tube defects.[27] The Institute of Medicine recommended these labels contain the most useful nutritional information for consumers: saturated fats, trans fats, sodium, calories, and serving size.[28] In January 2011, food manufacturers and grocery stores announced plans to display some of this nutrition information on processed food.[29]
The nutrition facts label currently appears on more than 6.5 billion food packages. President Bill Clinton issued an award of design excellence for the nutrition facts label in 1997 to Burkey Belser in Washington, DC.[30]
The FDA does not require any specific typeface be used in the Nutrition Facts label, mandating only that the label "utilize a single easy-to-read type style",[31] though its example label uses Helvetica.[32] However, as regulated by the FDA and the USDA, it is mandatory for certain information listed in the label to be written in English, including: name of the product, net quantity, serving size and number of servings per package, nutrition facts, ingredient list, and name of manufacturer or distributor.[33] The smallest lettering should be at least 1/16th of an inch tall (1.5875 millimeters), based on the height of a lowercase "o".[34]
In January 2006, Trans fat was required to be listed under saturated fat. This was the first significant change to the Nutrition Facts panel since it was introduced in 1993.[35]
2016 revision
In 2014, the U.S. Food and Drug Administration proposed several simultaneous improvements to nutrition labeling for the first time in over 20 years.[36][37] The proposed changes were based on trends of consumption of nutrients of public health importance.[38] However, studies had shown that the majority of the U.S. population could not understand the information in the then current Nutrition Facts Label.[39] Nutrition label numeracy is particularly low in older individuals, of black and Hispanic race/ethnicity, who are unemployed, born outside of the US, have lower English proficiency, lower education achievement, lower income, or live in the South.[40]
Proposed changes included a new design requiring serving sizes to more accurately reflect how many servings the average individual is actually consuming. The new recommendation also proposed removing “calories from fat”, and instead focusing on total calories and type of fats being consumed in a product. The proposed labels were to also list how much sugar is added (rather than inherent) to a product, as well as declaring the amount of Vitamin D and potassium in a product.[36][41] Some of these changes sparked a major debate between the food industry and public health agencies. The proposal to indicate sugar added during food production, in particular, was brought forward by the FDA as a measure to counter the increase in per capita sugar consumption in the US, which over the last decades exceeded the limits recommended by scientific institutions and governmental agencies.[42][43] Major American food associations opposed the label change, indicating "lack of merit" and "no preponderance of evidence" to justify the inclusion of sugar added in the new label.[44][45]
The rules for the new design were finalized on May 20, 2016. Manufacturers were initially given until July 26, 2018 to comply (or July 26, 2019 if they have less than $10 million in annual food sales).[46] A proposed rule by the FDA would extend the compliance deadline to January 1, 2020 (or January 1, 2021 for qualified small business).[47] For food and dietary supplement labeling purposes the amounts of vitamins and nutritionally essential minerals in a serving are expressed as a percent of Daily Value (%DV). Many of the definitions of 100% Daily Value were changed as part of the revision.[48] A table of the old and new adult Daily Values is provided at Reference Daily Intake.
Alcohol
In the United States, alcoholic beverages are regulated by the Alcohol and Tobacco Tax and Trade Bureau (TTB). As of 2012, the TTB does not require alcoholic beverage packaging to have a nutrition facts label. Since at least 2003, consumer groups have lobbied the TTB to require labelling disclosing Nutrition Facts information.[49] Some marketing terms, such as "light" and "table wine", must follow TTB guidelines. Packaging must disclose alcohol content in some circumstances.[49]
Mandatory information on the label varies by type of beverage, and includes:[50][51][52][53]
- Brand name
- Name and address of manufacturer (either bottling plant or main headquarters)
- Country of origin if imported (required by U.S. Customs and Border Protection regulations)
- Class, the definitions of which are regulated (e.g. beer, ale, lager, gin, vodka, rum, tequilla, cordial, liqueurs)
- Health warning for beverages 0.5% or more alcohol by volume
- Net contents
- For malt beverages, must be in United States customary units (e.g. pints or fluid ounces)
- For distilled spirits, must be in metric units. Bottles must be 50 mL, 100 mL, 200 mL, 375 mL, 750 mL, 1 L, or 1.75 L. Bottles must be 50 mL, 100 mL, 200 mL, or 355 mL.
- For wine, must be in metric units, and bottles must be 50 mL, 100 mL, 187 mL, 375 mL, 500 mL, 750 mL, 1 L, 1.5 L, 3 L, or a larger size with an even number of liters.
- Alcohol content (percent by volume):
- For malt beverages, mandatory only if some alcohol is due to added flavors, or if required by state law
- For distilled spirits, mandatory
- For wine, optional
- Declaration of sulfites required for wine sold in interstate (not intrastate) commerce if 10 ppm or more of sulfur dioxide
- Optional but regulated terms:
- For malt beverages: "draft", "light", "low-carbohydrate"
- For wine: grape variety and appellation of origin, wine designation (e.g. "white", "red", "rose", "table"), viticultural area, "estate bottled, "vinted", vintage date
- "Organic" (regulated by USDA)
- Declarations for major allergens
Caffeine
Health researchers have called for the mandatory labelling of food products with added caffeine, which is a psychoactive nervous system stimulant. If over-consumed, caffine can cause seizures, kidney problems, liver problems, heart arrhythmia, and death.[54] The Coca-Cola Company and PepsiCo began labelling caffeine content in 2007.[55]
See also
- Diet (nutrition)
- Food energy
- List of food labeling regulations
- Table of food nutrients
- Nutrition scale
- Serving size
- Atwater system (for calculating available food energy)
- The Non-GMO Project
- Quack Miranda warning
References
- "Nutrition Facts Label Images for Download". Fda.gov. 2011-09-23. Retrieved 2013-01-26.
- Food Standards Australia and New Zealand Standard 1.2.8 "Archived copy" (PDF). Archived from the original (PDF) on 2009-05-14. Retrieved 2009-01-03.CS1 maint: archived copy as title (link)
- "Greens welcome food labelling move". 3 News NZ. April 8, 2013.
- "Nutrition Labelling - Food and Nutrition - Health Canada". Hc-sc.gc.ca. 2004-07-26. Retrieved 2013-01-26.
- Directorate, Government of Canada, Canadian Food Inspection Agency, Food Labelling and Claims (2015-04-14). "Bilingual Labelling". www.inspection.gc.ca.
- "General Rules for Nutrition labeling of prepackaged foods" (PDF). USDA Foreign Agriculture Service. Retrieved 2018-03-16.
- "GB 28050-2011 (chinese)". www.gsciq.gov.cn. Retrieved 2018-03-16.
- "COMMISSION DIRECTIVE 2008/100/EC of 28 October 2008". EUR-Lex.europa.eu. Retrieved 2013-01-26.
- "REGULATION (EU) No 1169/2011 on the provision of food information to consumers".
- "REGULATION (EC) No 1924/2006 on nutrition and health claims made on foods".
- Commission Regulation (EU) No 1047/2012, European Commission, 8 November 2012, Retrieved 7 April 2015
- Commission Regulation (EU) No 1048/2012, European Commission, 8 November 2012, Retrieved 7 April 2015
- SANTE, DG. "Nutrition and Health Claims - European Commission". ec.europa.eu.
- Food Labelling Regulations 1996, Schedule 7 - Nutrition Labelling, The Stationery Office, 1996, retrieved 2009-04-04
- "Hong Kong government". Nutritionlabel.gov.hk. Retrieved 2014-08-01.
- "PFA Rule Relating to Nutritional Labeling of Packaged Food Implemented" (PDF). USDA Foreign Agricultural Service. Retrieved 24 November 2014.
- "India: Packaged foods must list nutritional facts". Freshplaza.com. Retrieved 2013-01-26.
- "Archived copy". Archived from the original on 2006-11-14. Retrieved 2007-01-29.CS1 maint: archived copy as title (link)
- "mexicolaws.com". mexicolaws.com. Retrieved 2013-01-26.
- CFR 101.9(c)8(iv) Archived 2011-06-13 at the Wayback Machine
- https://web.archive.org/web/20121031115637/http://www.crnusa.org/about_recs4.html. Archived from the original on 31 October 2012. Missing or empty
|title=(help) - See 21 CFR 101.9(c)(8) Archived 2009-08-13 at the Wayback Machine.
- VII. Nutrition Labeling; Questions G1 through P8. Guidance for Industry: A Food Labeling Guide. Accessed 2010-10-08. See also Guidance for Industry: Nutrition Labeling Manual - A Guide for Developing and Using Data Bases from the FDA.
- Code of Federal Regulations Title 21
- "Milestones in U.S. Food and Drug Law History". FDA. Archived from the original on 6 March 2013. Retrieved 11 February 2013.
- Saltos E, Davis C, et al. (December 1994). "Using Food Labels To Follow the Dietary Guidelines for Americans: A Reference". Agriculture Information Bulletin Number 704. United States Department of Agriculture, Center for Nutrition Policy and Promotion. Retrieved November 25, 2008.
- Wheeler, Madelyn; Marion Franz; Joan Heins; Rebecca Schafer; Harold Holler; et al. (May 1994). "Food Labeling" (PDF). Diabetes Care. 17 (5): 480–7. doi:10.2337/diacare.17.5.480. PMID 8062626. Retrieved 28 January 2014.
- "Examination of Front-of-Package Nutrition Rating Systems and Symbols: Phase 1 Report". Institute of Medicine. 2010-10-13. Archived from the original on 2011-01-11. Retrieved 2011-01-26.
- "Food Makers Devise Own Label Plan". New York Times. 2010-01-25. Retrieved 2011-01-26.
- "Briefs - The NIH Record". National Institutes of Health. 2006-04-27. Archived from the original on 2009-06-09. Retrieved 2009-06-16.
- "21 CFR 101.9(d)(1)(ii)(A)" (PDF).
- "Examples of Revised Nutrition Facts Panel Listing Trans Fat". U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition. 2003-07-09. Archived from the original on October 13, 2007. Retrieved 2007-11-08.
- Davidson, Tish (2008). "Food Labeling". The Gale Encyclopedia of Diets: A Guide to Health and Nutrition. 1: 407–412. Retrieved 23 January 2014.
- "21 CFR 101.2 - Information panel of package form food". www.gpo.gov.
- "Trans Fats Added To Nutrition Labels". MedicineNet. Retrieved 2016-04-15.
- "Proposed Changes to the Nutrition Facts Label". U.S. Food and Drug Administration. 1 August 2014. Retrieved 15 February 2015.
- "Nutrition Facts Label: Proposed Changes Aim to Better Inform Food Choices" (PDF). Consumer Health Information. US Food and Drug Administration. February 2014. Retrieved 15 February 2015.
- "Proposed Nutrition Facts Label Changes Are Based on Science and Research".
- Rothman, Russell L.; Housam, Ryan; Weiss, Hilary; Davis, Dianne; Gregory, Rebecca; Gebretsadik, Tebeb; Shintani, Ayumi; Elasy, Tom A. (2006-11-01). "Patient Understanding of Food Labels: The Role of Literacy and Numeracy". American Journal of Preventive Medicine. 31 (5): 391–398. doi:10.1016/j.amepre.2006.07.025. PMID 17046410.
- Nogueira, Leticia M.; Thai, Chan L.; Nelson, Wendy; Oh, April (2016-07-01). "Nutrition Label Numeracy: Disparities and Association with Health Behaviors". American Journal of Health Behavior. 40 (4): 427–436. doi:10.5993/AJHB.40.4.4. PMID 27338989.
- "FDA finalizes menu and vending machine calorie labeling rules". www.fda.gov. Food and Drug Administration. Retrieved 25 November 2014.
- Ferdman, Roberto A. (25 June 2014). "How the sugar lobby helps perpetuate that sweet tooth of yours". The Washington Post. Retrieved 15 February 2015.
- Weingus, Leigh (3 February 2015). "Here's Why Nutrition Labels Should List Added Sugar". The Huffington Post. Retrieved 15 February 2015.
- Ferdman, Roberto A. (2 July 2014). "The crucial FDA nutrition label battle you probably don't know about, but should". The Washington Post. Retrieved 15 February 2015.
- Prentice, Chris (4 August 2014). "Food fight builds as U.S. regulators weigh 'added sugar' label". Reuters. Retrieved 15 February 2015.
- Nutrition, Center for Food Safety and Applied (2019-06-18). "Labeling & Nutrition - Changes to the Nutrition Facts Label". FDA.
- Abram, Anna K. (October 2, 2017). "Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Proposed Extension of Compliance Dates". Regulations.gov. Retrieved October 3, 2017.
- "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF).
- Michelle Locke (2011-01-23). "Alcohol industry grapples with nutrition labeling". USA Today. Retrieved 2013-01-20.
- ALFD. "TTB - Advertising - Alcohol Beverage Labeling and Advertising". ttb.gov.
- "What You Should Know About Malt Beverage Labels" (PDF).
- "What You Should Know About Distilled Spirit Labels" (PDF).
- "What You Should Know About Grape Wine Labels" (PDF).
- Jon Kole1; Anne Barnhill (2013). "Caffeine Content Labeling: A Missed Opportunity for Promoting Personal and Public Health". Journal of Caffeine Research. 3 (3): 108–113. doi:10.1089/jcr.2013.0017. PMC 3777296. PMID 24761278.
- Elena Conis (December 28, 2009). "Labeling standards for caffeine". Los Angeles Times.
External links
| Wikimedia Commons has media related to Nutrition information. |
- US Food and Drug Administration Center for Food Safety and Applied Nutrition: Food labeling and nutrition
- Health Canada: Nutrition labeling
- U.K. Food Standards Agency: UK nutrition labelling
- EU website on nutrition labeling