Nootropic
Nootropics (/noʊ.əˈtrɒpɪks/ noh-ə-TROP-iks) (colloquial: smart drugs and cognitive enhancers) are drugs, supplements, and other substances that may improve cognitive function, particularly executive functions, memory, creativity, or motivation, in healthy individuals.[1] While many substances are purported to improve cognition, research is at a preliminary stage as of 2019, and the effects of the majority of these agents are not fully determined.
The use of cognition-enhancing drugs by healthy individuals in the absence of a medical indication spans numerous controversial issues, including the ethics and fairness of their use, concerns over adverse effects, and the diversion of prescription drugs for nonmedical uses, among others.[1][2] Nonetheless, the international sales of cognition-enhancing supplements has continued to grow over time, exceeding US$1 billion in 2015.[3]
In 2018 in the United States, some nootropic supplements were identified as having misleading ingredients and illegal marketing.[4][5] In 2019, the US Food and Drug Administration (FDA) and Federal Trade Commission (FTC) warned manufacturers and consumers about possible advertising fraud and marketing scams concerning nootropic supplements.[6][7]
Etymology
The term "nootropic" is derived from the Ancient Greek words νόος (nóos) meaning "mind" and τροπή (tropḗ) meaning "a turning".[8][9][10]
Availability and prevalence
In 2008, the most commonly used class of drug was stimulants, such as caffeine.[11] Manufacturers' marketing claims for dietary supplements are usually not formally tested and verified by independent entities.[12]
In 2016, the American Medical Association adopted a policy to discourage prescriptions of nootropics for healthy people on the basis that the effects of prescription stimulants are variable among individuals, dose-dependent, and modest.[13]
Use by students
The use of prescription stimulants is especially prevalent among students.[14] Surveys suggest that 0.7–4.5% of German students have used cognitive enhancers in their lifetimes.[15][16][17] Stimulants such as dimethylamylamine and methylphenidate are used on college campuses and by younger groups.[18] Based upon studies of self-reported illicit stimulant use, 5–35% of college students use diverted ADHD stimulants, which are primarily used for enhancement of academic performance rather than as recreational drugs.[19][20][21] Several factors positively and negatively influence an individual's willingness to use a drug for the purpose of enhancing cognitive performance. Among them are personal characteristics, drug characteristics, and characteristics of the social context.[15][16][22][23]
Side effects
The main concern with pharmaceutical drugs is adverse effects, which also apply to nootropics with undefined effects. Long-term safety evidence is typically unavailable for nootropics.[18] Racetams — piracetam and other compounds that are structurally related to piracetam — have few serious adverse effects and low toxicity, but there is little evidence that they enhance cognition in people having no cognitive impairments.[24]
In the United States, dietary supplements may be marketed if the manufacturer can show that the supplement is generally recognized as safe, and if the manufacturer does not make any claims about using the supplement to treat or prevent any disease or condition; supplements that contain drugs or advertise health claims are illegal under US law.[25]
Drugs
Central nervous system stimulants
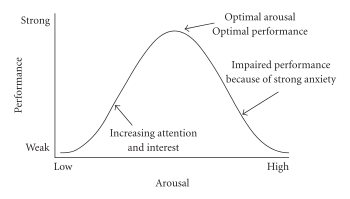
Systematic reviews and meta-analyses of clinical human research using low doses of certain central nervous system stimulants found that these drugs enhance cognition in healthy people.[26][27][28] In particular, the classes of stimulants that demonstrate cognition-enhancing effects in humans act as direct agonists or indirect agonists of dopamine receptor D1, adrenoceptor A2, or both types of receptor in the prefrontal cortex.[26][27][29][30] Relatively high doses of stimulants cause cognitive deficits.[29][30]
- Amphetamine – systematic reviews and meta-analyses report that low-dose amphetamine improve cognitive functions (e.g., inhibitory control, episodic memory, working memory, and aspects of attention) in healthy people and in individuals with ADHD.[26][27][28][30] A 2014 systematic review noted that low doses of amphetamine also improve memory consolidation, in turn leading to improved recall of information in non-ADHD youth.[28] It also improves task saliency (motivation to perform a task) and performance on tedious tasks that required a high degree of effort.[27][29][30]
- Methylphenidate – a benzylpiperidine that improves working memory, episodic memory, and inhibitory control, aspects of attention, and planning latency in healthy people.[26][27][28] It also may improve task saliency and performance on tedious tasks.[30] At above optimal doses, methylphenidate has off–target effects that decreased learning.[31]
- Eugeroics (armodafinil and modafinil) – are classified as "wakefulness-promoting agents"; modafinil increases alertness, particularly in sleep deprived individuals, and facilitates reasoning and problem solving in non-ADHD youth.[28] In a systematic review of small, preliminary studies where the effects of modafinil were examined, when simple psychometric assessments were considered, modafinil intake enhanced executive function.[32] Modafinil does not produce improvements in mood or motivation in sleep deprived or non-sleep deprived individuals.[33]
- Caffeine – a meta-analysis found an increase in alertness and attentional performance.[34][29]
- Nicotine – a meta-analysis of 41 clinical studies concluded that nicotine administration or smoking improves alerting and orienting attention and episodic and working memory and slightly improves fine motor performance.[35]
Racetams
Racetams, such as piracetam, oxiracetam, phenylpiracetam, and aniracetam, which are often marketed as cognitive enhancers and sold over-the-counter.[36] A recent study found that piracetam supplements sold in the United States were inaccurately labeled.[36] Racetams are often referred to as nootropics, but this property is not well established.[37] The racetams have poorly understood mechanisms, although piracetam and aniracetam are known to act as positive allosteric modulators of AMPA receptors and appear to modulate cholinergic systems.[38]
According to the US Food and Drug Administration,
"Piracetam is not a vitamin, mineral, amino acid, herb or other botanical, or dietary substance for use by humans to supplement the diet by increasing the total dietary intake. Further, piracetam is not a concentrate, metabolite, constituent, extract or combination of any such dietary ingredient. [...] Accordingly, these products are drugs, under section 201(g)(1)(C) of the Act, 21 U.S.C. § 321(g)(1)(C), because they are not foods and they are intended to affect the structure or any function of the body. Moreover, these products are new drugs as defined by section 201(p) of the Act, 21 U.S.C. § 321(p), because they are not generally recognized as safe and effective for use under the conditions prescribed, recommended, or suggested in their labeling."[39]
Miscellaneous
- Tolcapone – a systematic review noted that it improved verbal episodic memory and episodic memory encoding.[40]
- Levodopa – a systematic review noted that it improved verbal episodic memory and episodic memory encoding.[40]
- Atomoxetine – may improve working memory and attention when used at certain doses.[30]
- Pramipexole – no significant cognition-enhancing effects in healthy individuals.[40]
- Guanfacine – no significant cognition-enhancing effects in healthy individuals.[40]
- Clonidine – no significant cognition-enhancing effects in healthy individuals.[40]
- Fexofenadine – no significant cognition-enhancing effects in healthy individuals.[40]
Herbs
- Bacopa monnieri is used in Ayurvedic traditional medicine to improve memory.[41] Meta-analysis and reviews of clinical studies found evidence that the herb may improve cognition.[41][42][43]
- Panax ginseng – A review by the Cochrane Collaboration concluded that "there is a lack of convincing evidence to show a cognitive enhancing effect of Panax ginseng in healthy participants and no high quality evidence about its efficacy in patients with dementia."[44] According to the National Center for Complementary and Integrative Health, "[a]lthough Asian ginseng has been widely studied for a variety of uses, research results to date do not conclusively support health claims associated with the herb."[45]
- Ginkgo biloba – An extract of Ginkgo biloba leaf is marketed in dietary supplement form with claims it can enhance cognitive function in people without known cognitive problems, although there is no high-quality evidence to support such effects on memory or attention in healthy people.[46][47]
- Salvia officinalis (sage) – Some research has suggested certain extracts of Salvia officinalis may have positive effects on human brain function, but due to significant methodological problems, no firm conclusions can be drawn.[48][49] The thujone present in Salvia extracts may be neurotoxic.[49]
History
The term "nootropic" was coined by Corneliu Giurgea in 1972[9][10] to describe a new classification of molecules that acted selectively towards the brain's higher-level integrative activity.[50]
Nutrients and dietary supplements
A 2015 review found that use of omega-3 fatty acids, B vitamins, and vitamin E as nootropics was ineffective on cognitive function in normal middle-aged and older people.[51]
- Omega-3 fatty acids: DHA and EPA – two Cochrane Collaboration reviews on the use of supplemental omega-3 fatty acids for ADHD and learning disorders conclude that there is limited evidence of treatment benefits for either disorder.[52][53] Two other systematic reviews found no cognition-enhancing effects in the general population.[51][54]
- Folate – no cognition-enhancing effects in middle-aged and older adults without folate deficiency.[51]
- Vitamin B6 – no cognition-enhancing effects in middle-aged and older adults without B6 deficiency.[51]
- Vitamin B12 – no cognition-enhancing effects in middle-aged and older adults without B12 deficiency.[51]
- Vitamin E – no cognition-enhancing effects in middle-aged and older adults without vitamin E deficiency.[51]
- L-Theanine – A 2014 systematic review and meta-analysis found that concurrent caffeine and L-theanine use had synergistic psychoactive effects that promoted alertness, attention, and task switching;[34] these effects were most pronounced during the first hour post-dose.[34] However, the European Food Safety Authority reported that, when L-theanine is used by itself (i.e. without caffeine), there is insufficient information to determine if these effects exist.[55]
Society and culture
Illegal marketing claims
Because nootropic supplements are falsely advertised as unapproved drugs that were not proven to be effective for improving cognition in normal people and in those with cognitive disorders,[4] the FDA and FTC warned manufacturers and consumers in 2019 about possible advertising fraud and marketing scams concerning nootropic supplement products.[6][7][56][57] The FDA and FTC stated that some nootropic products had not been approved as a drug effective for any medical purpose, were not proven to be safe, and were illegally marketed in the United States under violation of the Federal Food, Drug, and Cosmetic Act.[6][7]
Over the years 2010 to 2019, the FDA warned numerous supplement manufacturers about the illegal status of their products as unapproved drugs with no proven safety or efficacy at the doses recommended, together with misleading marketing.[6][7][56][57][58][39]
See also
- Cognitive science
- Evidence-based learning
- Human enhancement
- List of drugs used by militaries
- Neurobiological effects of physical exercise § Persistent effects on cognition
- Neurobiological effects of physical exercise § Transient effects on cognition
- Neuroenhancement
- Neuroplastic effects of pollution
References
- Frati P, Kyriakou C, Del Rio A, Marinelli E, Vergallo GM, Zaami S, Busardò FP (January 2015). "Smart drugs and synthetic androgens for cognitive and physical enhancement: revolving doors of cosmetic neurology". Curr Neuropharmacol. 13 (1): 5–11. doi:10.2174/1570159X13666141210221750. PMC 4462043. PMID 26074739.
- Albertson TE, Chenoweth JA, Colby DK, Sutter ME (2016). "The Changing Drug Culture: Use and Misuse of Cognition-Enhancing Drugs". FP Essent. 441: 25–9. PMID 26881770.
- Chinthapalli K (September 2015). "The billion dollar business of being smart". BMJ. 351: h4829. doi:10.1136/bmj.h4829. PMID 26370589.
- Hank Schultz (May 17, 2018). "Some shady ingredients find home in nootropics category". NutraIngredients-USA.com, William Reed Business Media Ltd. Retrieved May 11, 2019.
- Markham Heid (January 23, 2019). "Nootropics, or 'Smart Drugs,' Are Gaining Popularity. But Should You Take Them?". Time. Retrieved May 12, 2019.
- "FTC and FDA Send Warning Letters to Companies Selling Dietary Supplements Claiming to Treat Alzheimer's Disease and Remediate or Cure Other Serious Illnesses Such as Parkinson's, Heart Disease, and Cancer". US Food and Drug Administration, US Federal Trade Commission. February 11, 2019. Retrieved May 11, 2019.
- "Health fraud scams: Unproven Alzheimer's disease products". US Food and Drug Administration. December 22, 2018. Retrieved May 11, 2019.
- "Definition of Nootropic by Lexico". Lexico Dictionaries | English. Retrieved November 4, 2019.
- Giurgea, Corneliu; Salama, M. (January 1, 1977). "Nootropic drugs". Progress in Neuro-Psychopharmacology. 1 (3): 235–247. doi:10.1016/0364-7722(77)90046-7. ISSN 0364-7722.
The term "nootropic" (noos = mind; tropein = towards) was proposed by us (Giurgea, 1972,1973) to designate psychotropic drugs
- Giurgea, Corneliu (1972). "Pharmacology of integrative activity of the brain. Attempt at nootropic concept in psychopharmacology". Actualités Pharmacologiques (Paris) (in French). 25: 115–156. PMID 4541214.
- Greely, Henry; Sahakian, Barbara; Harris, John; Kessler, Ronald C.; Gazzaniga, Michael; Campbell, Philip; Farah, Martha J. (December 10, 2008). "Towards responsible use of cognitive-enhancing drugs by the healthy". Nature. 456 (7223): 702–705. Bibcode:2008Natur.456..702G. doi:10.1038/456702a. ISSN 1476-4687. OCLC 01586310. PMID 19060880.
- "Dietary Supplements: What You Need to Know". US Food and Drug Administration. Retrieved February 14, 2015.
- "AMA confronts the rise of nootropics". American Medical Association. June 14, 2016. Retrieved May 12, 2019.
- McCabe, Sean Esteban; Knight, John R.; Teter, Christian J.; Wechsler, Henry (January 1, 2005). "Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey". Addiction. 100 (1): 96–106. doi:10.1111/j.1360-0443.2005.00944.x. hdl:2027.42/74786. PMID 15598197.
- Sattler, S.; Sauer, C.; Mehlkop, G.; Graeff, P. (2013). "The Rationale for Consuming Cognitive Enhancement Drugs in University Students and Teachers". PLoS ONE. 8 (7): e68821. Bibcode:2013PLoSO...868821S. doi:10.1371/journal.pone.0068821. PMC 3714277. PMID 23874778.
- Sattler, Sebastian; Wiegel, Constantin (February 25, 2013). "Cognitive Test Anxiety and Cognitive Enhancement: The Influence of Students' Worries on Their Use of Performance-Enhancing Drugs". Substance Use & Misuse. 48 (3): 220–232. doi:10.3109/10826084.2012.751426. PMID 23302063.
- Bossaer, John. "The Use and Misuse of Prescription Stimulants as "Cognitive Enhancers" by Students at One Academic Health Sciences Center". Academic Medicine. Archived from the original on April 10, 2015. Retrieved October 6, 2014.
- Sahakian B; Morein-Zamir S (December 2007). "Professor's little helper". Nature. 450 (7173): 1157–9. Bibcode:2007Natur.450.1157S. doi:10.1038/4501157a. PMID 18097378.
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ (October 2006). "Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration". Pharmacotherapy. 26 (10): 1501–1510. doi:10.1592/phco.26.10.1501. PMC 1794223. PMID 16999660.
- Weyandt LL, Oster DR, Marraccini ME, Gudmundsdottir BG, Munro BA, Zavras BM, Kuhar B (September 2014). "Pharmacological interventions for adolescents and adults with ADHD: stimulant and nonstimulant medications and misuse of prescription stimulants". Psychol. Res. Behav. Manag. 7: 223–249. doi:10.2147/PRBM.S47013. PMC 4164338. PMID 25228824.
- Clemow DB, Walker DJ (September 2014). "The potential for misuse and abuse of medications in ADHD: a review". Postgrad. Med. 126 (5): 64–81. doi:10.3810/pgm.2014.09.2801. PMID 25295651.
- Sattler, Sebastian; Mehlkop, Guido; Graeff, Peter; Sauer, Carsten (February 1, 2014). "Evaluating the drivers of and obstacles to the willingness to use cognitive enhancement drugs: the influence of drug characteristics, social environment, and personal characteristics". Substance Abuse Treatment, Prevention, and Policy. 9 (1): 8. doi:10.1186/1747-597X-9-8. ISSN 1747-597X. PMC 3928621. PMID 24484640.
- Sattler, Sebastian; Forlini, Cynthia; Racine, Éric; Sauer, Carsten (August 5, 2013). "Impact of Contextual Factors and Substance Characteristics on Perspectives toward Cognitive Enhancement". PLOS ONE. 8 (8): e71452. Bibcode:2013PLoSO...871452S. doi:10.1371/journal.pone.0071452. ISSN 1932-6203. LCCN 2006214532. OCLC 228234657. PMC 3733969. PMID 23940757.
- Malykh AG, Sadaie MR (February 2010). "Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders". Drugs. 70 (3): 287–312. doi:10.2165/11319230-000000000-00000. PMID 20166767.
- Goldman P (2001). "Herbal medicines today and the roots of modern pharmacology". Annals of Internal Medicine. 135 (8 Pt 1): 594–600. doi:10.7326/0003-4819-135-8_Part_1-200110160-00010. PMID 11601931.
- Spencer RC, Devilbiss DM, Berridge CW (June 2015). "The Cognition-Enhancing Effects of Psychostimulants Involve Direct Action in the Prefrontal Cortex". Biol. Psychiatry. 77 (11): 940–950. doi:10.1016/j.biopsych.2014.09.013. PMC 4377121. PMID 25499957.
- Ilieva IP, Hook CJ, Farah MJ (January 2015). "Prescription Stimulants' Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis". J. Cogn. Neurosci. 27 (6): 1069–89. doi:10.1162/jocn_a_00776. PMID 25591060.
- Bagot KS, Kaminer Y (April 2014). "Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: a systematic review". Addiction. 109 (4): 547–557. doi:10.1111/add.12460. PMC 4471173. PMID 24749160.
- Wood S, Sage JR, Shuman T, Anagnostaras SG (January 2014). "Psychostimulants and cognition: a continuum of behavioral and cognitive activation". Pharmacol. Rev. 66 (1): 193–221. doi:10.1124/pr.112.007054. PMC 3880463. PMID 24344115.
- Malenka RC, Nestler EJ, Hyman SE, Holtzman DM (2015). "Chapter 14: Higher Cognitive Function and Behavioral Control". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (3rd ed.). New York: McGraw-Hill Medical. ISBN 9780071827706.
- Urban, KR; Gao, WJ (2014). "Performance enhancement at the cost of potential brain plasticity: neural ramifications of nootropic drugs in the healthy developing brain". Frontiers in Systems Neuroscience. 8: 38. doi:10.3389/fnsys.2014.00038. PMC 4026746. PMID 24860437.
- Battleday, R.M.; Brem, A.-K. (November 2015). "Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review" (PDF). European Neuropsychopharmacology. 25 (11): 1865–1881. doi:10.1016/j.euroneuro.2015.07.028. ISSN 0924-977X. PMID 26381811.
- Meulen, Ruud ter; Hall, Wayne; Mohammed, Ahmed (2017). Rethinking Cognitive Enhancement. Oxford University Press. p. 116. ISBN 9780198727392.
- Camfield DA, Stough C, Farrimond J, Scholey AB (2014). "Acute effects of tea constituents L-theanine, caffeine, and epigallocatechin gallate on cognitive function and mood: a systematic review and meta-analysis". Nutr. Rev. 72 (8): 507–22. doi:10.1111/nure.12120. PMID 24946991.
- Heishman SJ, Kleykamp BA, Singleton EG (June 2010). "Meta-analysis of the acute effects of nicotine and smoking on human performance". Psychopharmacology. 210 (4): 453–69. doi:10.1007/s00213-010-1848-1. PMC 3151730. PMID 20414766.
- Cohen, Pieter A.; Zakharevich, Igor; Gerona, Roy (November 25, 2019). "Presence of Piracetam in Cognitive Enhancement Dietary Supplements". JAMA Internal Medicine. doi:10.1001/jamainternmed.2019.5507.
- Malenka RC, Nestler EJ, Hyman SE (2009). Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 454. ISBN 9780071481274.
- Gualtieri F, Manetti D, Romanelli MN, Ghelardini C (2002). "Design and study of piracetam-like nootropics, controversial members of the problematic class of cognition-enhancing drugs". Curr. Pharm. Des. 8 (2): 125–38. doi:10.2174/1381612023396582. PMID 11812254.
- John Gridley (August 30, 2010). "FDA Warning Letter: Unlimited Nutrition". Office of Compliance, Center for Food Safety and Applied Nutrition, Inspections, Compliance, Enforcement, and Criminal Investigations, US Food and Drug Administration. Retrieved April 5, 2016.
- Fond G, Micoulaud-Franchi JA, Brunel L, Macgregor A, Miot S, Lopez R, Richieri R, Abbar M, Lancon C, Repantis D (September 2015). "Innovative mechanisms of action for pharmaceutical cognitive enhancement: A systematic review". Psychiatry Res. 229 (1–2): 12–20. doi:10.1016/j.psychres.2015.07.006. PMID 26187342.
- Aguiar, Sebastian; Borowski, Thomas (2013). "Neuropharmacological review of the nootropic herb Bacopa monnieri". Rejuvenation Research. 16 (4): 313–326. doi:10.1089/rej.2013.1431. ISSN 1557-8577. PMC 3746283. PMID 23772955.
- Kongkeaw, C; Dilokthornsakul, P; Thanarangsarit, P; Limpeanchob, N; Norman Scholfield, C (2014). "Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract". Journal of Ethnopharmacology. 151 (1): 528–35. doi:10.1016/j.jep.2013.11.008. PMID 24252493.
- Neale, Chris; Camfield, David; Reay, Jonathon; Stough, Con; Scholey, Andrew (February 5, 2013). "Cognitive effects of two nutraceuticals Ginseng and Bacopa benchmarked against modafinil: a review and comparison of effect sizes". British Journal of Clinical Pharmacology. 75 (3): 728–737. doi:10.1111/bcp.12002. ISSN 0306-5251. PMC 3575939. PMID 23043278.
- Geng J, Dong J, Ni H, Lee MS, Wu T, Jiang K, Wang G, Zhou AL, Malouf R (2010). "Ginseng for cognition". Cochrane Database Syst Rev (12): CD007769. doi:10.1002/14651858.CD007769.pub2. PMID 21154383.
- "Asian Ginseng". National Center for Complementary and Integrative Health, US National Institutes of Health. September 2016. Retrieved July 9, 2018.
- Laws KR, Sweetnam H, Kondel TK (November 2012). "Is Ginkgo biloba a cognitive enhancer in healthy individuals? A meta-analysis". Hum Psychopharmacol. 27 (6): 527–33. doi:10.1002/hup.2259. PMID 23001963.
- "Ginkgo". National Center for Complementary and Integrative Health, US National Institutes of Health. September 2016. Retrieved July 9, 2018.
- Miroddi M, Navarra M, Quattropani MC, Calapai F, Gangemi S, Calapai G (2014). "Systematic review of clinical trials assessing pharmacological properties of Salvia species on memory, cognitive impairment and Alzheimer's disease". CNS Neurosci Ther. 20 (6): 485–95. doi:10.1111/cns.12270. PMC 6493168. PMID 24836739.
- Lopresti AL (2017). "Salvia (Sage): A Review of its Potential Cognitive-Enhancing and Protective Effects". Drugs in R&D. 17 (1): 53–64. doi:10.1007/s40268-016-0157-5. PMC 5318325. PMID 27888449.
- Giurgea, Corneliu E. (1982). "The nootropic concept and its prospective implications". Drug Development Research. 2 (5): 441–446. doi:10.1002/ddr.430020505.
The nootropic concept emerged about 10 years ago essentially from the unusual pharmacology of piracetam, which later on was confirmed and extended to human pharmacoclinics and therapeutics. A nootropic drug is characterized by a direct functional activation of the higher integrative brain mechanisms that enhances cortical vigilance, a telencephalic functional selectivity, and a particular efficiency in restoring deficient higher nervous activity.
- Forbes SC, Holroyd-Leduc JM, Poulin MJ, Hogan DB (December 2015). "Effect of nutrients, dietary supplements and vitamins on cognition: a systematic review and meta-analysis of randomized controlled trials". Canadian Geriatrics Journal. 18 (4): 231–45. doi:10.5770/cgj.18.189. PMC 4696451. PMID 26740832.
- Gillies D; Sinn JKh; Lad SS; Leach MJ; Ross MJ (2012). "Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents". Cochrane Database Syst Rev. 7 (7): CD007986. doi:10.1002/14651858.CD007986.pub2. PMC 6599878. PMID 22786509.
- Tan ML, Ho JJ, Teh KH (September 28, 2016). "Polyunsaturated fatty acids (PUFAs) for children with specific learning disorders". Cochrane Database Syst Rev. 12: CD009398. doi:10.1002/14651858.CD009398.pub2. PMID 23235675.
- Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P (2015). "Omega-3 polyunsaturated fatty acid supplementation and cognition: A systematic review and meta-analysis" (PDF). J. Psychopharmacol. (Oxford). 29 (7): 753–63. doi:10.1177/0269881115587958. PMID 26040902.
- EFSA Panel on Dietetic Products, Nutrition and Allergies; European Food Safety Authority (EFSA), Parma, Italy (2011). "Scientific Opinion on the substantiation of health claims related to L-theanine from Camellia sinensis (L.) Kuntze (tea) and improvement of cognitive function (ID 1104, 1222, 1600, 1601, 1707, 1935, 2004, 2005), alleviation of psychological stress (ID 1598, 1601), maintenance of normal sleep (ID 1222, 1737, 2004) and reduction of menstrual discomfort (ID 1599) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal. 9 (6): 2238. doi:10.2903/j.efsa.2011.2238.CS1 maint: multiple names: authors list (link)
- William A Correll, Jr. (February 5, 2019). "FDA Warning Letter: Peak Nootropics LLC aka Advanced Nootropics". Office of Compliance, Center for Food Safety and Applied Nutrition, Inspections, Compliance, Enforcement, and Criminal Investigations, US Food and Drug Administration. Retrieved May 11, 2019.
- William A Correll, Jr. (February 5, 2019). "FDA Warning Letter: TEK Naturals". Office of Compliance, Center for Food Safety and Applied Nutrition, Inspections, Compliance, Enforcement, and Criminal Investigations, US Food and Drug Administration. Retrieved May 11, 2019.
- Emma R. Singleton (January 7, 2010). "FDA Warning Letter: Cerebral Health LLC". Office of Compliance, Center for Food Safety and Applied Nutrition, Inspections, Compliance, Enforcement, and Criminal Investigations, US Food and Drug Administration. Retrieved May 12, 2019.
External links
