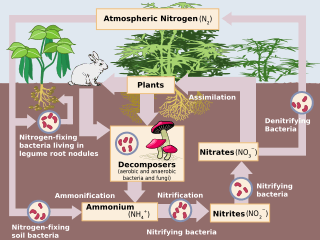
Nitrification
Nitrification is the biological oxidation of ammonia to nitrite followed by the oxidation of the nitrite to nitrate.[1] The transformation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an important step in the nitrogen cycle in soil. Nitrification is an aerobic process performed by small groups of autotrophic bacteria and archaea. This process was discovered by the Russian microbiologist Sergei Winogradsky.
Microbiology and ecology
The oxidation of ammonia into nitrite is performed by two groups of organisms, ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA[2]).[3] AOB can be found among the β-proteobacteria and gammaproteobacteria.[4] Currently, two AOA, Nitrosopumilus maritimus and Nitrososphaera viennensis, have been isolated and described.[5] In soils the most studied AOB belong to the genera Nitrosomonas and Nitrosococcus. Although in soils ammonia oxidation occurs by both AOB and AOA, AOA dominate in both soils and marine environments,[2][6][7] suggesting that Thaumarchaeota may be greater contributors to ammonia oxidation in these environments.[2]
The second step (oxidation of nitrite into nitrate) is done (mainly) by bacteria of the genus Nitrobacter and Nitrospira. Both steps are producing energy to be coupled to ATP synthesis. Nitrifying organisms are chemoautotrophs, and use carbon dioxide as their carbon source for growth. Some AOB possess the enzyme, urease, which catalyzes the conversion of the urea molecule to two ammonia molecules and one carbon dioxide molecule. Nitrosomonas europaea, as well as populations of soil-dwelling AOB, have been shown to assimilate the carbon dioxide released by the reaction to make biomass via the Calvin Cycle, and harvest energy by oxidizing ammonia (the other product of urease) to nitrite. This feature may explain enhanced growth of AOB in the presence of urea in acidic environments.[8]
In most environments, organisms are present that will complete both steps of the process, yielding nitrate as the final product. However, it is possible to design systems in which nitrite is formed (the Sharon process).
Nitrification is important in agricultural systems, where fertilizer is often applied as ammonia. Conversion of this ammonia to nitrate increases nitrogen leaching because nitrate is more water-soluble than ammonia.
Nitrification also plays an important role in the removal of nitrogen from municipal wastewater. The conventional removal is nitrification, followed by denitrification. The cost of this process resides mainly in aeration (bringing oxygen in the reactor) and the addition of an external carbon source (e.g., methanol) for the denitrification.
Nitrification can also occur in drinking water. In distribution systems where chloramines are used as the secondary disinfectant, the presence of free ammonia can act as a substrate for ammonia-oxidizing microorganisms. The associated reactions can lead to the depletion of the disinfectant residual in the system.[9] The addition of chlorite ion to chloramine-treated water has been shown to control nitrification.[10][11]
Together with ammonification, nitrification forms a mineralization process that refers to the complete decomposition of organic material, with the release of available nitrogen compounds. This replenishes the nitrogen cycle.
Chemistry and enzymology
Nitrification is a process of nitrogen compound oxidation (effectively, loss of electrons from the nitrogen atom to the oxygen atoms), and is catalyzed step-wise by a series of enzymes.
OR
In Nitrosomonas europaea, the first step of oxidation (ammonia to hydroxylamine) is carried out by the enzyme ammonia monooxygenase (AMO).
The second step (hydroxylamine to nitrite) is carried out step-wise by two different enzymes. Hydroxylamine oxidoreductase (HAO), converts hydroxylamine to nitric oxide.[12]
Another as-of-yet unknown enzyme that converts nitric oxide to nitrite.
The third step (nitrite to nitrate) is completed in a different organism.
Nitrification in the marine environment
In the marine environment, nitrogen is often the limiting nutrient, so the nitrogen cycle in the ocean is of particular interest.[13][14] The nitrification step of the cycle is of particular interest in the ocean because it creates nitrate, the primary form of nitrogen responsible for "new" production. Furthermore, as the ocean becomes enriched in anthropogenic CO2, the resulting decrease in pH could lead to decreasing rates of nitrification. Nitrification could potentially become a "bottleneck" in the nitrogen cycle.[15]
Nitrification, as stated above, is formally a two-step process; in the first step ammonia is oxidized to nitrite, and in the second step nitrite is oxidized to nitrate. Different microbes are responsible for each step in the marine environment. Several groups of ammonia-oxidizing bacteria (AOB) are known in the marine environment, including Nitrosomonas, Nitrospira, and Nitrosococcus. All contain the functional gene ammonia monooxygenase (AMO) which, as its name implies, is responsible for the oxidation of ammonia.[2][14] More recent metagenomic studies have revealed that some Thaumarchaeota (formerly Crenarchaeota) possess AMO. Thaumarchaeotes are abundant in the ocean and some species have a 200 times greater affinity for ammonia than AOB, leading researchers to challenge the previous belief that AOB are primarily responsible for nitrification in the ocean.[13] Furthermore, though nitrification is classically thought to be vertically separated from primary production because the oxidation of nitrogen by bacteria is inhibited by light, nitrification by AOA does not appear to be light inhibited, meaning that nitrification is occurring throughout the water column, challenging the classical definitions of "new" and "recycled" production.[13]
In the second step, nitrite is oxidized to nitrate. In the oceans, this step is not as well understood as the first, but the bacteria Nitrospina and Nitrobacter are known to carry out this step in the sea.[13]
Soil conditions controlling nitrification rates
- Substrate availability (presence of NH4+)
- Aeration (availability of O2)
- Well-drained soils with 60% soil moisture
- pH (near neutral)
- Temperature (best 20-30 °C) => Nitrification is seasonal, affected by land use practices
Inhibitors of nitrification
Nitrification inhibitors are chemical compounds that slow the nitrification of ammonia, ammonium-containing, or urea-containing fertilizers, which are applied to soil as fertilizers. These inhibitors can help reduce losses of nitrogen in soil that would otherwise be used by crops. Nitrification inhibitors are used widely, being added to approximately 50% of the fall-applied anhydrous ammonia in states in the U.S., like Illinois.[16] They are usually effective in increasing recovery of nitrogen fertilizer in row crops, but the level of effectiveness depends on external conditions and their benefits are most likely to be seen at less than optimal nitrogen rates.[17]
The environmental concerns of nitrification also contribute to interest in the use of nitrification inhibitors: the primary product, nitrate, leaches into groundwater, producing acute toxicity in multiple species of wildlife and contributing to the eutrophication of standing water. Some inhibitors of nitrification also inhibit the production of methane, a greenhouse gas.
The inhibition of the nitrification process is primarily facilitated by the selection and inhibition/destruction of the bacteria that oxidize ammonia compounds. A multitude of compounds that inhibit nitrification, which can be divided into the following areas: the active site of ammonia monooxygenase (AMO), mechanistic inhibitors, and the process of N-heterocyclic compounds. The process for the latter of the three is not yet widely understood, but is prominent. The presence of AMO has been confirmed on many substrates that are nitrogen inhibitors such as dicyandiamide, ammonium thiosulfate, and nitrapyrin.
The conversion of ammonia to hydroxylamine is the first step in nitrification, where AH2 represents a range of potential electron donors.
- NH3 + AH2 + O2 → NH2OH + A + H2O
This reaction is catalyzed by AMO. Inhibitors of this reaction bind to the active site on AMO and prevent or delay the process. The process of oxidation of ammonia by AMO is regarded with importance due to the fact that other processes require the co-oxidation of NH3 for a supply of reducing equivalents. This is usually supplied by the compound hydroxylamine oxidoreductase (HAO) which catalyzes the reaction:
- NH2OH + H2O → NO2− + 5 H+ + 4 e−
The mechanism of inhibition is complicated by this requirement. Kinetic analysis of the inhibition of NH3 oxidation has shown that the substrates of AMO have shown kinetics ranging from competitive to noncompetitive. The binding and oxidation can occur on two different locations on AMO: in competitive substrates, binding and oxidation occurs at the NH3 site, while in noncompetitive substrates it occurs at another site.
Mechanism based inhibitors can be defined as compounds that interrupt the normal reaction catalyzed by an enzyme. This method occurs by the inactivation of the enzyme via covalent modification of the product, which ultimately inhibits nitrification. Through the process, AMO is deactivated and one or more proteins is covalently bonded to the final product. This is found to be most prominent in a broad range of sulfur or acetylenic compounds.
Sulfur-containing compounds, including ammonium thiosulfate (a popular inhibitor) are found to operate by producing volatile compounds with strong inhibitory effects such as carbon disulfide and thiourea.
In particular, thiophosphoryl triamide has been a notable addition where it has the dual purpose of inhibiting both the production of urease and nitrification.[18] In a study of inhibitory effects of oxidation by the bacteria Nitrosomonas europaea, the use of thioethers resulted in the oxidation of these compounds to sulfoxides, where the S atom is the primary site of oxidation by AMO. This is most strongly correlated to the field of competitive inhibition.
N-heterocyclic compounds are also highly effective nitrification inhibitors and are often classified by their ring structure. The mode of action for these compounds is not well understood: while nitrapyrin, a widely used inhibitor and substrate of AMO, is a weak mechanism-based inhibitor of said enzyme, the effects of said mechanism are unable to correlate directly with the compound’s ability to inhibit nitrification. It is suggested that nitrapyrin acts against the monooxygenase enzyme within the bacteria, preventing growth and CH4/NH4 oxidation.[19] Compounds containing two or three adjacent ring N atoms (pyridazine, pyrazole, indazole) tend to have a significantly higher inhibition effect than compounds containing non-adjacent N atoms or singular ring N atoms (pyridine, pyrrole).[20] This suggests that the presence of ring N atoms is directly correlated with the inhibition effect of this class of compounds.
Methane inhibition
Some enzymatic nitrification inhibitors, such as urease, can also inhibit the production of methane in methanotrophic bacteria. AMO shows similar kinetic turnover rates to methane monooxygenase (MMO) found in methanotrophs, indicating that MMO is a similar catalyst to AMO for the purpose of methane oxidation. Furthermore, methanotrophic bacteria share many similarities to NH
3 oxidizers such as Nitrosomonas.[21] The inhibitor profile of particulate forms of MMO (pMMO) shows similarity to the profile of AMO, leading to similarity in properties between MMO in methanotrophs and AMO in autotrophs.
Environmental concerns
Nitrification inhibitors are also of interest from an environmental standpoint because of the production of nitrates and nitrous oxide from the process of nitrification. Nitrous oxide (N2O), although its atmospheric concentration is much lower than that of CO2, has a global warming potential of about 300 times greater than carbon dioxide and contributes 6% of planetary warming due to greenhouse gases. This compound is also notable for catalyzing the breakup of ozone in the stratosphere.[22] Nitrates, a toxic compound for wildlife and livestock and a product of nitrification, are also of concern.
Soil, consisting of polyanionic clays and silicates, generally has a net anionic charge. Consequently, ammonium (NH4+) binds tightly to the soil but nitrate ions (NO3−) do not. Because nitrate is more mobile, it leaches into groundwater supplies through agricultural runoff. Nitrates in groundwater can affect surface water concentrations, either through direct groundwater-surface water interactions (e.g., gaining stream reaches, springs), or from when it is extracted for surface use. As an example, much of the drinking water in the United States comes from groundwater, but most wastewater treatment plants discharge to surface water.
Wildlife such as amphibians, freshwater fish, and insects are sensitive to nitrate levels, and have been known to cause death and developmental anomalies in affected species.[23] Nitrate levels also contribute to eutrophication, a process in which large algal blooms reduce oxygen levels in bodies of water and lead to death in oxygen-consuming creatures due to anoxia. Nitrification is also thought to contribute to the formation of photochemical smog, ground level ozone, acid rain, changes in species diversity, and other undesirable processes. In addition, nitrification inhibitors have also been shown to suppress the oxidation of methane (CH4), a potent greenhouse gas, to CO2. Both nitrapyrin and acetylene are shown to be especially strong suppressors of both processes, although the modes of action distinguishing them are unclear.
See also
- f-ratio
- Haber process
- Nitrifying bacteria
- Nitrogen fixation
- Simultaneous nitrification-denitrification
- Comammox
References
- Nitrification Network. "Nitrification primer". nitrificationnetwork.org. Oregon State University. Retrieved 21 August 2014.
- Hatzenpichler, R (2012). "Diversity, physiology and niche differentiation of ammonia-oxidizing archaea". Appl Environ Microbiol. 78 (21): 7501–7510. doi:10.1128/aem.01960-12. PMC 3485721. PMID 22923400.
- Treusch, A. H.; Leininger, S.; Kletzin, A.; Schuster, S. C.; Klenk, H. P.; Schleper, C. (2005). "Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling". Environmental Microbiology. 7 (12): 1985–95. doi:10.1111/j.1462-2920.2005.00906.x. PMID 16309395.
- Purkhold, U.; Pommerening-Roser, A.; Juretschko, S.; Schmid, M.C.; Koops, H.-P.; Wagner, M. (2000). "Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implications for molecular diversity surveys". Appl Environ Microbiol. 66 (12): 5368–5382. doi:10.1128/aem.66.12.5368-5382.2000. PMC 92470. PMID 11097916.
- Martens-Habbena, W.; Berube, P. M.; Urakawa, H.; de la Torre, J. R.; Stahl, D. A. (2009). "Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria". Nature. 461 (7266): 976–981. doi:10.1038/nature08465. PMID 19794413.
- Wuchter, C.; Abbas, B.; Coolen, M.J.L.; Herfort, L.; van Bleijswijk, J.; Timmers, P.; et al. (2006). "Archaeal nitrification in the ocean". Proc Natl Acad Sci USA. 103 (33): 12317–12322. doi:10.1073/pnas.0600756103. PMC 1533803. PMID 16894176.
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G. W.; Prosser, J. I.; Schuster, S. C.; Schleper, C. (2006). "Archaea predominate among ammonia-oxidizing prokaryotes in soils" (PDF). Nature. 442 (7104): 806–809. doi:10.1038/nature04983. PMID 16915287.
- Marsh, K. L.; Sims, G. K.; Mulvaney, R. L. (2005). "Availability of urea to autotrophic ammonia-oxidizing bacteria as related to the fate of 14C- and 15N-labeled urea added to soil". Biol. Fert. Soil. 42 (2): 137–145. doi:10.1007/s00374-005-0004-2.
- Zhang, Y; Love, N; Edwards, M (2009). "Nitrification in Drinking Water Systems". Critical Reviews in Environmental Science and Technology. 39 (3): 153–208. doi:10.1080/10643380701631739.
- McGuire, Michael J.; Lieu, Nancy I.; Pearthree, Marie S. (1999). "Using chlorite ion to control nitrification". Journal - American Water Works Association. 91 (10): 52–61. doi:10.1002/j.1551-8833.1999.tb08715.x.
- McGuire, Michael J.; Wu, Xueying; Blute, Nicole K.; Askenaizer, Daniel; Qin, Gang (2009). "Prevention of nitrification using chlorite ion: Results of a demonstration project in Glendale, Calif". Journal - American Water Works Association. 101 (10): 47–59. doi:10.1002/j.1551-8833.2009.tb09970.x.
- Caranto, Jonathan D.; Lancaster, Kyle M. (2017-08-01). "Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase". Proceedings of the National Academy of Sciences. 114 (31): 8217–8222. doi:10.1073/pnas.1704504114. PMC 5547625. PMID 28716929.
- Zehr, J. P.; Kudela, R. M. (2011). "Nitrogen cycle of the open ocean: From genes to ecosystems". Annual Review of Marine Science. 3: 197–225. doi:10.1146/annurev-marine-120709-142819. PMID 21329204.
- Ward, B.B. (1996). "Nitrification and denitrification: Probing the nitrogen cycle in aquatic environments" (PDF). Microbial Ecology. 32 (3). doi:10.1007/BF00183061.
- Hutchins, David; Mulholland, Margaret; Fu, Feixue (2009). "Nutrient cycles and marine microbes in a CO2-enriched ocean". Oceanography. 22 (4): 128–145. doi:10.5670/oceanog.2009.103.
- Czapar, George F.; Payne, Jean; Tate, Jodie (2007). "An Educational Program on the Proper Timing of Fall-applied Nitrogen Fertilizer". Cm. 6: 0. doi:10.1094/CM-2007-0510-01-RS.
- Ferguson, R; Lark, R; Slater, G. (2003). "Approaches to management zone definition for use of nitrification inhibitors". Soil Sci. Soc. Am. J. 67: 937–947. doi:10.2136/sssaj2003.9370 (inactive 2019-08-20).
- McCarty, G. W. (1999). "Modes of action of nitrification inhibitors". Biology and Fertility of Soils. 29: 1–9. doi:10.1007/s003740050518.
- Topp, E; Knowles, R (1984). "Effects of Nitrapyrin [2-Chloro-6-(trichloromethyl) Pyridine] on the Obligate Methanotroph Methylosinus trichosporium OB3b". Appl. Environ. Microbiol. 47 (2): 258–262. doi:10.1007/BF01576048.
- McCarty, G.W. (1998). "Modes of action of nitrification inhibitors". Biology and Fertility of Soils. 29 (1): 1–9. doi:10.1007/s003740050518.
- Knowles, B (1989). "Physiology, biochemistry and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers". Microbiol. Rev. 53 (1): 68–84. PMC 372717. PMID 2496288.
- Singh, S. N.; Verma, Amitosh (2007). "Environmental Review: The Potential of Nitrification Inhibitors to Manage the Pollution Effect of Nitrogen Fertilizers in Agricultural and Other Soils: A Review". Environmental Practice. 9 (4): 266–279. doi:10.1017/S1466046607070482.
- Rouse, J; Bishop, C; Struger, J (1999). "Nitrogen pollution: an assessment of its threat to amphibian survival". Environ. Health Perspect. 107 (10): 799–803. doi:10.2307/3454576. JSTOR 3454576. PMC 1566592. PMID 10504145.
External links
- Nitrification at the heart of filtration at fishdoc.co.uk
- Nitrification at University of Aberdeen · King's College
- Nitrification Basics for Aerated Lagoon Operators at lagoonsonline.com