Neonatal seizure
A neonatal seizure is a seizure in a baby younger than 4 weeks old. Seizures are abnormally excessive or synchronous neuronal activity in the brain. They are the most frequent neurological problem in the nursery, and often require evaluation and treatment in a neonatal intensive care unit. Seizures in the neonatal population can be categorized into acute symptomatic seizures and genetic or structural causes. Diagnosis relies on identification of the cause of the seizure, and verification of actual seizure activity by measuring of electrical activity with electroencephalography (EEG). Treatment depends on the cause of the seizure, but often includes pharmacologic treatment with anti-epileptic drugs.
| Neonatal seizure | |
|---|---|
| Other names | Seizures in neonates |
Considerable debate about the longterm consequence of a neonatal seizure exists between data and deductions reached through animal experimentations and those obtained through clinical investigations. The main conflicting issues are whether seizures in newborns can plant the roots for epileptogenesis and cause long-term deficits.[1] Fewer than half of the affected infants develop seizures in later life. Such neonatal seizures are considered self-limited, and thus the term neonatal epilepsy is not used to describe these seizures. It has been estimated that approximately 15% of neonatal seizures represent an epilepsy syndrome.[2] The incidence of neonatal seizures has not been clearly established, although an estimated frequency of 80-120 cases per 100,000 neonates per year has been suggested. The incidence of seizures is higher in the neonatal period than at any other time of life,[3] and most often occurs in the first week of life.[4]
Signs and symptoms
Seizures in the neonatal population often present differently than in other age groups due to brain immaturity. Electroclinical seizures are defined by evidence of seizure activity on electroencephalogram as well as clinical signs or symptoms. However, in neonates there may be no clinical movement abnormality (either because the seizures are subclinical or because they were not witnessed), so the only symptom may be abnormal level of consciousness. When motor movements due occur, they cannot be suppressed by gentle restraint by a nurse or caregiver. Classification systems have been developed based on neonatal seizure motor manifestations, summarized below.[5] For more information about seizure classification, see page seizure types.
Focal or multifocal clonic
Clonic seizures are defined by repetitive contractions of groups of muscles, typically of the limbs or face. These may involve one group of muscles (focal) or the multiple groups of muscles (multifocal). An isolated focal seizure can move or spread, and can even alternate from one side of the body to the other. Due to the neonatal brain's immaturity, the typical Jacksonian march may not occur. Focal seizures typically have very close correlates on EEG, with measurable EEG abnormalities with each seizure movement. The rhythm of the clonic movements and EEG abnormalities is usually slow, at 1-3 movements per second.
Focal tonic
Focal tonic seizures are characterized by sustained muscle contraction of muscle groups, and thus often appear as abnormal posturing of a single limb or eye deviation.
Generalized tonic
A focal tonic seizure can generalize, or the first seizure can occur as a generalized seizure, or seizures that impair the neonate's level of consciousness. Generalized tonic seizures typically appear as symmetric and sustained posturing of limbs in either an extensor or flexor distribution. Generalized tonic seizures often manifest with tonic extension of the upper and lower limbs and also may involve the axial musculature in an opisthotonic fashion. Generalized tonic seizures mimic decorticate posturing; the majority are not associated with electrographic seizures.
Myoclonic
Myoclonic movements can either be caused by seizures or be benign neonatal sleep myoclonus, a common mimicker of seizures in neonates (see differential diagnosis, below). Myoclonic seizures are characterized by isolated and fast contractions of muscle groups. These movements typically occur in the limbs or face, and do not repeat. Stimulation can provoke myoclonic seizures.
Subtle
Some clinicians use the term subtle seizures to describe seizures if there is an absence of distinct tonic or clonic movements but presence of abnormal eye movements, stereotyped lip smacking, or apneic events.
Benign
Benign neonatal seizures are not classified as epilepsy.[6]
Causes
Neonatal seizures have a number of causes. Determining the cause of a confirmed seizure is important because treatment and prognosis vary based on underlying etiology of the seizure. In contrast to seizures that occur in other age groups, seizures that occur during the neonatal period are most often caused by the following processes:
- Hypoxic-Ischemic Encephalopathy: This is the most common cause of seizures in the neonatal period.[7]
- Perinatal Arterial Stroke: Arterial stroke can be caused by intra-arterial thrombosis or embolism from the heart or placenta. The risk for perinatal arterial stroke increases with a variety of conditions that occur due to material factors during birth (oligohydramnios, chorioamnionitis, placental abnormalities) or neonatal factors (clotting disorders, congenital heart defects)[7]
- Intraventricular hemorrhage
- Central Nervous System Infection: CNS Infection are found in 3-10% of neonates who seize, though exact incidence varies between studies.[7] Bacterial meningitis and viral meningoencephalitis are most prevalent, though fungal infections can occur.
- Congenital central nervous system malformations: Lissencephaly, polymicrogyria, and tuberous sclerosis are specific entities known to cause seizures due to defects in brain tissue development. Since prognosis is poor and often these disorders are genetic, identification of this etiology is of utmost importance to be able to counsel parents appropriately.
- Inborn Errors of Metabolism: Inborn errors of metabolism can cause physiologic conditions that result in seizures. These errors are genetic and often are accompanied by other symptoms such as lethargy, poor feeding, and low tone.[7] Diagnosis often involves specific laboratory tests of metabolic products as well as genetic tests. Several classification systems exist for seizures caused by inborn errors of metabolism, one of which separates causes into problems with neurotransmitter metabolism, energy production, and biosynthetic substances crucial for brain formation.[8]
- Electrolyte abnormalities: Metabolic abnormalities such as hypoglycemia, hyponatremia, and hypocalcemia can manifest as seizures.
- Substance-Related: Neonatal abstinence syndrome occurs when maternal drug use before birth results in a fetal withdrawal syndrome. Substances include alcohol, cocaine, narcotics, tricyclclic antidepressants, or other sedatives. Seizures can be prevented from occurring if the symptoms of withdrawal are recognized and treated early.[7]
Diagnosis
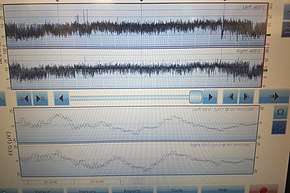
Seizure activity in a neonate is difficult to diagnose, as many seizures have no clinical correlate. Altered level of consciousness is often the only clue, and in a neonate this can be difficult to accurately assess. Thus, diagnosis relies on attempting to directly measurement the abnormal electrical activity in the brain with electroencephalography (EEG). EEG is combined with video recording of the infant to correlate any seizure movements with EEG recordings.
There are several modes of EEG that are commonly used in the NICU to diagnose neonatal seizures. Conventional continuous multichannel conventional EEG is the gold standard for diagnosis of epileptiform activity, but requires expert interpretation. Newer amplitude integrated EEG (aEEG) (also termed cerebral function monitoring, or CFM) allows easier monitoring of brain activity, but may not allow identification of short duration, low amplitude, or very high frequency seizure activity.[9] Often, both modes are displayed concurrently.
Determining the cause
Since many causes of seizures can be rapidly reversed and longterm sequelae prevented, evaluation of underlying cause is of utmost importance. Evaluation for infection (with blood counts, lumbar puncture, and empiric treatment with antibiotics) often occurs during EEG monitoring. Blood glucose and electrolyte testing can identify metabolic problems that can be corrected. Further testing includes evaluation for genetic causes and other more rare metabolic causes.[10] Brain injury such as cerebral infarction or hemorrhage can be evaluated with imaging techniques such as magnetic resonance imaging (MRI) and brain ultrasound to avoid unnecessary radiation exposure.
Differential diagnosis
Infants can exhibit stereotyped movements that may be hard to distinguish from seizure activity. Since many of these non-seizure movements are not dangerous and require no treatment, differentiation from actual seizure activity is useful. Jitteriness is common in the neonatal period and is seen in upwards of 2/3 of neonates. It is characterized by a tremor that is especially prominent during sleep or periods of agitation. Gaze deviation or eye movements do not occur. Benign neonatal sleep myoclonus (BNSM) is another common movement that can be mistaken for a seizure. It is characterized by jerking limb movements only during sleep, and stop with waking of the infant. BNSM typically occurs later in infancy, but can occur during the neonatal period.
Treatment
Once diagnosis is made, the goals of management are to identify the cause of the seizure, stop the seizure activity, and maintain physiologic parameters such as oxygenation, ventilation, blood glucose, and temperature.
Treatment greatly depends on the cause of the seizure. For example, infectious causes of seizures (meningitis, meningoencephalitis), are often treated with antimicrobials (antibiotic, antifungal, or antiviral medications). Similarly, electrolyte or glucose abnormalities are treated by repleting or lowering the offending electrolyte or sugar.
If the cause of the seizures are unlikely to be easily or quickly corrected, once diagnosis of a seizure is made, the mainstay of treatment is pharmacotherapy with anti-epileptic drugs. A 2013 systematic review found that most practitioners use phenobarbital or phenytoin.[11] This study found that phenobarbital has the safest side effect profile and longest history of use in neonates. Benzodiazepines are often used as second line treatment if treatment with phenobarbital does not result in clinical improvement. Research is ongoing on use of other anti-epileptics that are commonly used in older children and adults are safe or efficacious to use in neonates. Part of the challenge of anticonvulsant drug treatment during the neonatal period is that the immature excitatory and inhibitory neurotransmitter system results in few effective drug targets.[10]
Outcomes
With earlier diagnosis and increasingly prompt diagnosis, mortality after diagnosis of neonatal seizures has decreased dramatically from an estimated 33% in the 1990s to around 10% in the 2010s.[12] Underlying cause of the seizure remains the greatest predictor of ongoing seizures and neurologic problems later in life. Controversy remains with the extent of damage the seizures themselves cause. Clinician consensus is that frequent or intractable seizures (status epilepticus) leads to neuronal damage and are associated with later neurodevelopment problems.[13]
Developmental delay, cerebral palsy, and problems with vision and hearing are the most common poor outcomes following neonatal seizures.[12] Severity of impairment ranges greatly and many infants develop normally once the initial seizure cause is treated.
Studies have identified risk factors for poor outcomes after neonatal seizures. Infants that are premature, have hypoxemic ischemic encephalopathy, CNS infection, severe intraventricular hemorrhage, structural central nervous system defect, or severely abnormal EEG tracings tend to do worse than infants with focal strokes, transient metabolic issues (hypoglycemia, hypocalcemia), or clinical seizures without EEG abnormalities.[12]
Risk of epilepsy later during childhood is a common question asked by parents in the neonatal intensive care unit (NICU). Unfortunately, the answer is rarely clear cut as it depends greatly on the underlying cause. For all infants with neonatal seizures regardless of cause, the rate of subsequent seizures during childhood is estimated between 10 and 20%.[14] Infants who survive severe global HIE have the highest rate of epilepsy later in life.[12]
Epidemiology
It is difficult to determine the incidence of seizures in the neonatal period. Estimations range between 1-5 per 1,000 live-births,[10] though the actual rate of seizures during this period may be higher due to lack of accurate diagnosis of sub-clinical seizure activity without continuous EEG monitoring. Acute causes of seizures (hypoxemic ischemic encephalopathy, infection, intracranial hemorrhage, stroke, etc) are more common than first-episode of neonatal epilepsy syndromes.
Seizures occur during the neonatal period at a higher rate than in any other period of life. Seizures in the developing brain are more common than in a mature brain for several reasons. First, the developing brain is hyperexcitable due to excess in excitatory glutaminergic neurons and immaturity of inhibitory gamma-amino butyric acid (GABA) neurons. Preterm infants are at especially high risk for seizures for this reason.[12]
Research directions
Since interpretation of continuous EEG monitoring requires a trained neurologist, automated interpretation software has been proposed. Algorithms and machine learning have been studied, however logistical and mathematic challenges remain.
See also
References
- Lombroso CT (2007). "Neonatal seizures: gaps between the laboratory and the clinic". Epilepsia. 48 Suppl 2: 83–106. doi:10.1111/j.1528-1167.2007.01070.x. PMID 17571356.
- Shellhaas RA, Wusthoff CJ, Tsuchida TN, Glass HC, Chu CJ, Massey SL, Soul JS, Wiwattanadittakun N, Abend NS, Cilio MR (August 2017). "Profile of neonatal epilepsies: Characteristics of a prospective US cohort". Neurology. 89 (9): 893–899. doi:10.1212/WNL.0000000000004284. PMC 5577964. PMID 28733343.
- Sheth RD, Hobbs GR, Mullett M (January 1999). "Neonatal seizures: incidence, onset, and etiology by gestational age". Journal of Perinatology. 19 (1): 40–3. doi:10.1038/sj.jp.7200107. PMID 10685200.
- Plu, Fred (1983). "Status epilepticus. Mechanisms of brain damage and treatment". Advances in Neurology. 34 (6): 1–551. doi:10.1002/ana.410150628. PMID 6829325.
- Scher, Mark S. (December 1998). "Diagnosis and management of neonatal seizures.By Eli M. Mizrahi and Peter Kellaway Philadelphia, Lippincott-Raven, 1998 192 pp, illustrated, $79.00". Annals of Neurology. 44 (6): 988. doi:10.1002/ana.410440628. ISSN 0364-5134.
- National Institute for Health and Clinical Excellence (January 2012). "9" (PDF). The Epilepsies: The diagnosis and management of the epilepsies in adults and children in primary and secondary care. National Clinical Guideline Centre. pp. 119–129.
- Vasudevan C, Levene M (August 2013). "Epidemiology and aetiology of neonatal seizures". Seminars in Fetal & Neonatal Medicine. 18 (4): 185–91. doi:10.1016/j.siny.2013.05.008. PMID 23746578.
- Van Hove JL, Lohr NJ (November 2011). "Metabolic and monogenic causes of seizures in neonates and young infants". Molecular Genetics and Metabolism. 104 (3): 214–30. doi:10.1016/j.ymgme.2011.04.020. PMID 21839663.
- Boylan GB, Stevenson NJ, Vanhatalo S (August 2013). "Monitoring neonatal seizures". Seminars in Fetal & Neonatal Medicine. 18 (4): 202–8. doi:10.1016/j.siny.2013.04.004. PMID 23707519.
- Glass HC (March 2014). "Neonatal seizures: advances in mechanisms and management". Clinics in Perinatology. 41 (1): 177–90. doi:10.1016/j.clp.2013.10.004. PMC 3925308. PMID 24524454.
- Slaughter LA, Patel AD, Slaughter JL (March 2013). "Pharmacological treatment of neonatal seizures: a systematic review". Journal of Child Neurology. 28 (3): 351–64. doi:10.1177/0883073812470734. PMC 3805825. PMID 23318696.
- Uria-Avellanal C, Marlow N, Rennie JM (August 2013). "Outcome following neonatal seizures". Seminars in Fetal & Neonatal Medicine. 18 (4): 224–32. doi:10.1016/j.siny.2013.01.002. PMID 23466296.
- Clancy RR, Legido A (February 1991). "Postnatal epilepsy after EEG-confirmed neonatal seizures". Epilepsia. 32 (1): 69–76. doi:10.1111/j.1528-1157.1991.tb05614.x. PMID 1985832.
- Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, Volpe J, Bourgeois B, du Plessis AJ (April 2006). "The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants". Pediatrics. 117 (4): 1270–80. doi:10.1542/peds.2005-1178. PMID 16585324.