Negative-sense single-stranded RNA virus
A negative-sense single-stranded RNA virus (or (-)ssRNA virus) is a virus that uses negative sense, single-stranded RNA as its genetic material. Single stranded RNA viruses are classified as positive or negative depending on the sense or polarity of the RNA. The negative viral RNA is complementary to the mRNA and must be converted to a positive RNA by RNA polymerase before translation. Therefore, the purified RNA of a negative sense virus is not infectious by itself, as it needs to be converted to a positive sense RNA for replication. These viruses belong to Group V on the Baltimore classification.[1]
| Negative ssRNA Virus | |
|---|---|
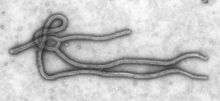 | |
| Ebola Virus | |
| Virus classification | |
| Group: | Group V ((−)ssRNA) |
| Order, Family, and Genus | |
| |
In addition, negative-sense single-stranded RNA viruses have complex genomic sequences, cell cycles, and replication habits that use various protein complexes to arrange in specific conformations and carry out necessary processes for survival and reproduction of their genomic sequences. The complexity of negative-sense single-stranded RNA viruses carries into its ability to suppress the innate immune response of the cells it infects and the construction of a capsid, which is unique to the varying classifications of negative-sense single-stranded RNA viruses.
Replication
Negative sense ssRNA viruses need RNA polymerase to form a positive sense RNA. The positive-sense RNA acts as a viral mRNA, which is translated into proteins for the production of new virion materials. With the newly formed virions, more negative sense RNA molecules are produced.
In more details, replication of the virion consists of the following steps:[2][3][4]
- A virion enters the host cell and releases its negative RNA into the cytoplasm.
- The virus uses its own RNA replicase, also known as RNA-dependent RNA polymerase (RdRp), to form positive RNA template strands through complementary base pairing.
- The positive RNA acts as mRNA, which is translated into structural capsomere proteins and viral RdRp by the host's ribosomes.
- A replicative complex is formed with RdRp: The positive strands can either function as mRNA to produce more proteins or as template to make more negative RNA strands.
- New viral capsids are assembled with the capsomere proteins. The negative RNA strands combine with capsids and viral RdRp to form new negative RNA virions.
- After assembly and maturation of nucleocapsid, the new virions exit the cell by budding or lysing through cell membrane to further infect other cells.
The genome size of a negative RNA virus is between 10kb to 30kb. Two genome subgroups can be distinguished, nonsegmented and segmented, and are described as such:
- In viruses with nonsegmented genomes, the first step of replication is transcription of the negative strand by RdRp to form various monocistronic mRNA that code for individual viral proteins. A positive strand copy is formed to serve as template for the production of the negative genome. This replication takes place in the cytoplasm.
- In viruses with segmented genomes, replication occurs in the nucleus and the RdRp produces one monocistronic mRNA strand from each genome segment. The principal difference between the two types is the location of replication.
Taxonomy
One phylum, two subphyla, six classes, eight orders and twenty one families are currently recognised in this group.[5] A number of unassigned species and genera are yet to be classified.[6] Outside the all encompassing Negarnaviricota phylum there is only Deltavirus genus.
- Phylum Negarnaviricota[7]
- Subphylum Haploviricotina
- Class Chunqiuviricetes
- Order Muvirales
- Family Qinviridae
- Order Muvirales
- Class Milneviricetes
- Order Serpentovirales
- Family Aspiviridae
- Order Serpentovirales
- Class Monjiviricetes
- Order Jingchuvirales
- Order Mononegavirales
- Family Bornaviridae — Borna disease virus
- Family Filoviridae — includes Ebola virus, Marburg virus
- Family Mymonaviridae
- Family Nyamiviridae[8]
- Family Paramyxoviridae — includes Measles virus, Mumps virus, Nipah virus, Hendra virus, and NDV
- Family Pneumoviridae — includes RSV and Metapneumovirus
- Family Rhabdoviridae — includes Rabies virus
- Family Sunviridae
- Genus Anphevirus
- Genus Arlivirus
- Genus Chengtivirus
- Genus Crustavirus
- Genus Wastrivirus
- Class Yunchangviricetes
- Order Goujianvirales
- Family Yueviridae
- Order Goujianvirales
- Class Chunqiuviricetes
- Subphylum Polyploviricotina
- Class Ellioviricetes
- Order Bunyavirales
- Family Arenaviridae — includes Lassa virus
- Family Feraviridae
- Family Fimoviridae
- Family Hantaviridae
- Family Jonviridae
- Family Nairoviridae
- Family Peribunyaviridae
- Family Phasmaviridae
- Family Phenuiviridae
- Family Tospoviridae
- Order Bunyavirales
- Class Insthoviricetes
- Order Articulavirales
- Family Amnoonviridae—includes Taastrup virus
- Family Orthomyxoviridae—includes Influenza viruses
- Order Articulavirales
- Class Ellioviricetes
- Subphylum Haploviricotina
- Unassigned genera:
- Genus Deltavirus — includes Hepatitis D virus
Host range
Viruses of the families Arenaviridae, Orthomyxoviridae, Paramyxoviridae, and Pneumoviridae are able to infect vertebrates. Viruses of the families Bunyaviridae and Rhabdoviridae are able to infect vertebrates, arthropods, and plants. Viruses of the genus Tenuivirus only infect plants. A few viruses known to infect humans include Marburg virus, Ebola, measles, mumps, rabies, and influenza.
- Lassa virus (Arenaviridae)
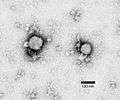 Lymphocytic choriomeningitis virus (Arenaviridae)
Lymphocytic choriomeningitis virus (Arenaviridae)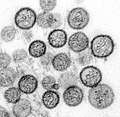 Hantavirus (Bunyaviridae)
Hantavirus (Bunyaviridae)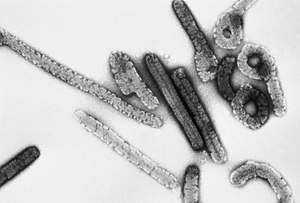 Marburg Virus (Filoviridae)
Marburg Virus (Filoviridae) Ebola virus (Filoviridae)
Ebola virus (Filoviridae)
- Measles (Paramyxoviridae)
 Mumps virus (Paramyxoviridae)
Mumps virus (Paramyxoviridae)_EM_PHIL_2175_lores.jpg) Human respiratory syncytial virus (Paramyxoviridae)
Human respiratory syncytial virus (Paramyxoviridae) Parainfluenza (Paramyxoviridae)
Parainfluenza (Paramyxoviridae)_EM_18_lores.jpg) Vesicular stomatitis virus (Rhabdoviridae)
Vesicular stomatitis virus (Rhabdoviridae)
Genome
The genome for negative-stranded RNA virus (NSV) consists of one to several single-stranded RNAs, which are assembled into complexes.[9] These complexes function as templates for the transcription and replication of the NSV genome by RNA polymerase. The NSV genome has been observed as being either segmented and nonsegmented.[9] The segmented genome can be divided up into anywhere between 2-8 RNA molecules.[9] A distinctive feature of the NSV genome is its highly structured organization. Multiple monomers of either the ribonucleoprotein (RNP) complexes or nucleocapsids associate with the RNA to create these organized complexes.[9] Nonsegmented RNA follows a sequential pattern of gene expression.[9] With segmented RNA, each individual segment is contained in a distinct RNP complex.[9] These complexes function independently for transcription and replication related processes.[9]
NSV ribonucleoproteins (RNPs) generally adopt a helical conformation.[9] For nonsegmented NSVs, these conformations are typically linear in fashion, and are relatively rigid.[10][11] Segmented NSVs are present in a more flexible, circular conformation.[12] This results from non-covalent RNA-RNA interactions between the 5’- and 3’-termini of the RNA segments.[13] Regarding the helical character of NSV RNPs, the main determinant of this conformation is the NP/N protein.[9] The structure of virion full-length RNPs are typically presented as a left-handed, double-helical arrangement of two NP strands that are opposite in polarity.[9] Recombinant RNPs have a different structure, generally consisting of a right-handed helix.[14] Nevertheless, wild type RNPs isolated from virus present left-handed helix [15]
Catalytic activity related to RNA synthesis is associated with a very large protein, termed the L protein. This is a multi-enzymatic polypeptide that is responsible for multiple tasks. These activities span multiple domains, including mRNA synthesis/modification and the formation of ribonucleoproteins.[16][17] Through phylogenetic analysis, it was determined that NSV RNA polymerases share a common ancestor with other RNA polymerases from various origins.[9] This is further supported by various L proteins displaying highly conserved sequence blocks that are in series, yet separated by variable regions.[18]
When discussing the NSV RNP, the NP/N protein is considered to be the most abundant element of the ribonucleoprotein.[9] These elements are what provide the basis for the RNPs helical conformation.[9] NP/N proteins are also vital for the transcription and replication of full-length RNA templates.[9] The proper function of NP/N proteins rely on their capacity to oligomerize. This defines the ability of NP/N proteins to complex together to form larger structural elements. The oligomerization mechanism is unique between the nonsegmented NSV genome (nsNSV) and segmented NSV genome (sNSV).[9] For nsNSVs, interactions occur between the protein monomers, while the stabilization of this complex occurs through inter-monomeric contact involving N-terminal and/or C-terminal protein extensions.[11][19][20] This contributes to the viral RNP helical structure.[10][11][21] Oligomerization for sNSVs has been shown to be similar to this for some Bunyaviridae, but it may also take place much more flexibly, defined by their ability to form much smaller oligomers.[22][9] These include, but are not limited to, dimers, trimers, tetramers, long helices, and more complex structures.[9]
NSV Life Cycle and Replication
Negative-strand RNA viruses (NSV) can be classified into 21 distinct families. The families consisting of nonsegmented genomes include Rhabdo-, Paramyxo-, Filo- and Borna-. Orthomyxo-, Bunya-, Arenaviridae- contain genomes of six to eight, three, or two negative-sense RNA segments, respectively.[23] Many highly prevalent human pathogens such as the respiratory syncytial virus (RSV), parainfluenza viruses, influenza viruses, Ebola virus, Marburg virus are included within the NSV. The life cycle of NSV has a number of steps. The virus first infects the host cell by binding to the host cell receptor through a viral surface glycoprotein.[24] The fusion of the glycoprotein viral membrane with the plasma membrane of the host cell in an acidic environment allows for the release of viral ribonucleoprotein (RNP) complexes into the cytoplasm. Most NSV replicate in the cytoplasm of infected cells. Newly synthesized RNP complexes are assembled with viral structural proteins at the plasma membrane or at membranes of the Golgi apparatus.[23] This is all followed by the release of the newly synthesized viruses.
In regards to the replication and transcription of non-segmented NSV, the genes of these NSV are made up of three regulatory regions: a gene end signal, an intergenic region, and a gene start signal.[23][25] One example of gene end signals are in a specific virus called the vesicular stomatitis virus (VSV) contains gene end signals that are highly conservative. The intergenic region is highly variable and consists of conserved dinucleotide, trinucleotide, or regions of up to 143 nucleotides.[25] The various lengths of the intergenic regions correlate with transcriptional attenuation, however diverse intergenic regions do not alter the gene expression. The gene start signals are highly specific as the first three nucleotides are critical for the gene expression.[23][25][26]
Molecular Mechanisms of Innate Antiviral Immune Inhibition
A cell’s innate immune response is the first line of defense for finding viral infections. The innate immune response triggers the production of type I interferons (IFN) and pro-inflammatory cytokines.[27][28] However, non-segmented negative sense RNA viruses (NNSV) have developed multifunctional proteins that can diffuse this innate response pathway to avoid antiviral functions within the cell.[28] This mainly occurs through the NNSV's proteins interacting with other cellular proteins involved in the type I interferon signaling pathway.[27] Due to the expansive nature of the immune pathway, NNSVs have a variety of areas throughout the signaling cascade that can be targeted for interruption of the production of type I interferons or pro-inflammatory cytokines.[29] Such areas include inhibiting the induction of the IFN pathway or inhibiting the response from the IFN signaling cascade.
NNSV-Mediated Inhibition of IFN Induction
NNSVs target several induction pathway areas, as outlined in the image, to avoid detection within the cell, or even inhibit that specific area of the signaling cascade. One such example of NNSVs avoiding cellular detection arises with mutations, which sequester the pathogen-associated molecular pathways.[27][30] By creating a mutation within the nucleotide sequence, specifically ones important for binding double stranded DNA or other proteins, the virus is able to go undetected by cell, avoid activation of the cellular antiviral response, and evade the immune response.
NNSVs can also bind to cellular receptors throughout the pro-inflammatory cytokine pathway to inhibit the immune response.[27] By carrying accessory proteins that directly bind to pattern recognition receptors, the virus can use its accessory proteins to induce conformational changes throughout other immune response proteins and inhibit cellular responses.[29] Generally, the pattern recognition receptors detect infection-associated molecules commonly associated with viruses, but some viruses carry accessory proteins that reconfigure the protein to inhibit its function and block the rest of the signaling cascade that would produce an immune response.
Other areas of inhibition of induction apply similar concepts of binding to cellular proteins and inhibiting their function throughout the immune cascade.[27][28] These include binding to proteins involved in dephosphorylation pathways and blocking DNA binding transcription factors. In each case, the accessory proteins coded for in the nucleotide sequence inhibit a critical function of other innate cellular proteins, disrupting the signaling cascade for producing type I interferons and pro-inflammatory cytokines.
NNSV-Mediated Inhibition of IFN Response
Another way that NSVs avoid the host immune response is to encode for proteins that target the JAK/STAT pathway or the nuclear transports mechanisms for transcription factors. Each of these are a portion of the IFN pathway described previously in the immune system’s innate response to viral infection.
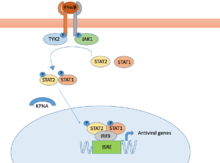
For the JAK/STAT pathway depicted in the image, a critical reaction for inducing the pathway is the proper phosphorylation of the TYK2 and JAK1 proteins.[27] Upon phosphorylation, the rest of the STAT pathway will begin and lead to the production of antiviral genes.[31] NNSVs have the capability to synthesize proteins that target the phosphorylation step of the pathway. By preventing the phosphorylation of TYK2 and JAK1, they stop the IFNα/β pathway and bring the antiviral immune response to a halt.[32] Similarly, viruses can also synthesize proteins that prevent the phosphorylation of STAT1 a little further along the signaling cascade. This process halts the IFNα/β pathway, just like preventing phosphorylation of TYK2 and JAK1.
Further down the IFNα/β pathway, STAT1 and STAT2 are transported across the nuclear membrane, as depicted in the image.[27][32] They accordingly bind to the DNA sequence and behave as transcription factors- affecting the level of gene production throughout the cell. In normal cellular function, this pathway will behave normally in response to viral infection, leading to the production of antiviral genes and the induction of an immune response.[27] However, NNSVs have developed the capability to generate complexes that target the protein responsible for translocating STAT1 across the nuclear membrane. By binding to and inhibiting this function, STAT1 is never able to bind to the DNA and properly regulate the production of antiviral genes.[31][32] This pathway of inhibiting the IFN response helps the NNSV go undetected within the cell and avoid certain immune response pathways.
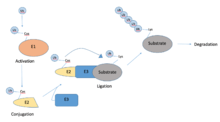
Ubiquitination
Another important process used throughout many cellular processes is ubiquitination—which is outlined in the image below. Many cells use this to locate and identify viruses, and to restrict the viral infection.[33] NNSVs however have developed a pathway to synthesize proteins that target the ubiquitin pathway along many of the signaling cascades descriptive of the IFN response. More specifically, the NNSVs are capable of reprogramming the host cell’s ubiquitination pathway in such a way that leads to the degradation of host cell mechanism that would otherwise silence a viral infection.[34]
Common Mechanism for RNA Encapsidation by NSVs
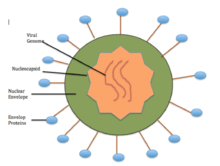
Like all viruses, negative-sense RNA viruses (NSVs) contain a protein capsid that encapsulates the genomic material. The nucleocapsid of NSVs is assembled with a single nucleocapsid protein, and the viral RNA. Each NSV nucleocapsid is packaged inside a lipid envelope, but the appearance of the nucleocapsid differs from virus to virus.[35] For example, in rhabdoviruses, the nucleocapsid assumes a bullet shape,[21] while in paramyxoviruses, the nucleocapsid is filamentous or herringbone-like.[36] However, when the nucleocapsids are released from the virion, they all appear like a coil.[35]
So far, the atomic structures of nucleocapsid-like-particles (NLP) have been elucidated for three NSV families: Rhabdoviridae, Paramyxoviridae, and Bunyaviridae. One important element of NSVs is that the capsid protein (N) is first synthesized as a monomeric protein (N0). N0 is a capsid protein that assembles a capsid to accommodate any RNA sequence. N0 is kept monomeric in different ways depending on which family of NSVs a virus falls into. For instance, rhabdoviruses keep the N0 monomeric by forming a complex with the phosphoprotein (P).[21] It has been found that both the N- and C terminal regions of P bind to the capsid protein. Essentially, the P binding occupies the site required for N0 oligomerization. Other viruses, such as bunyaviruses, simply sequester the N terminus by N itself. Nonetheless, it is essential for N to be monomeric for the NSV to be competent in encapsidating viral RNA.[21]
In terms of the structure of the nucleocapsid, the N protein will eventually oligomerize to encapsidate the single-stranded RNA. In some NSVs the N subunits are oriented in a parallel orientation, and the ssRNA is sequestered in the center. In most NSVs, the nucleocapsid appears to be a random coil, and the symmetry is linear.[37] Because the N subunits are oriented in a parallel fashion, the cross-molecular interactions among the subunits stabilize the nucleocapsid and are critical for capsid formation. The linear interactions along the encapsidated single-stranded RNA are actually a unique feature to NSVs.[21] X-ray crystal structures of N proteins and EM micrographs of RNP complexes from a number of Bunyaviridae (a Family of segmented NSVs that includes the Bunyamwera virus and the Schmallenberg virus) show that in these viruses the nucleocapsid adopts a helical arrangement where the N proteins form a head-to-tail chain by linking to each other via a flexible N-terminal arm. The resulting chain forms tight coils with four N proteins per turn that holds the circular ssRNA of these viruses on the inside of the coils and extend to form large circular filaments.[22]
The process of encapsidation is a concomitant process with viral replication, likely at the site of the viral RNA replication process.[38] Another unique feature of NSVs is that the sequestered bases are stacked to form a motif similar to half of the A-form double helix of RNA. The stacking arrangement allows for maximized packaging of the RNA in the capsid.[21] The base stacking may actually contribute to the overall stability of the nucleocapsid, and is dependent on the RNA sequence (i.e. poly(rA) is the most stable).[39] An interesting finding is that specific sequences in the sequestered RNA genome may regulate viral functions. For translational termination, there is a U7 track at the end of each coding region, causing this to be the least stable region. This therefore promotes dissociation of the transcription complex.[21] A unique aspect of NSVs is the conserved (3H+5H) motif, which has been identified to constitute the RNA cavity through secondary structure elements of the N protein.[40]
Lastly, a main distinction between NSVs from other viruses is that the nucleocapsid actually serves as the template for viral RNA synthesis. During synthesis, the nucleocapsid undergoes a conformational change to release the RNA template. After transcription is complete, the RNA is repositioned in the cavity, and the nucleocapsid is restored.[21]
See also
- Positive-sense single-stranded RNA virus
- Double-stranded RNA virus
- Baltimore classification
- Sense (molecular biology)
References
- Baltimore, D (1971). "Expression of animal virus genomes". Bacteriological Reviews. 35 (3): 235–241. PMC 378387. PMID 4329869.
- Samuel, Charles E (2005). "Virus-Host Interaction Minireview Series: Human Immunodeficiency Virus, Hepatitis C Virus, and Influenza Virus". The Journal of Biological Chemistry. 281 (13): 8305–7. doi:10.1074/jbc.R500032200. PMID 16407181.
- Hofkin, Bruce (2010). Living in a Microbial World. Garland Science. ISBN 9780815341758.
- Rybicki, Ed. "Viral Replication Strategy in Negative-Sense Single-Stranded RNA Viruses". ASM Microbe Library. American Society for Microbiology. Archived from the original on 23 October 2014. Retrieved 14 April 2015.
- Cann, Alan (2011). Principles of Molecular Virology. Academic Press. ISBN 978-0-12-384939-7.
- Klein, Donald W.; Prescott, Lansing M.; Harley, John (1993). Microbiology. Dubuque, Iowa: Wm. C. Brown. ISBN 978-0-697-01372-9.
- "Virus Taxonomy: 2018 Release". International Committee on Taxonomy of Viruses. Retrieved 13 November 2018.
- Mihindukulasuriya, K. A.; Nguyen, N. L.; Wu, G.; Huang, H. V.; Travassos da Rosa, A. P.; Popov, V. L.; Tesh, R. B.; Wang, D. (2009). "Nyamanini and Midway viruses define a novel taxon of RNA viruses in the order Mononegavirales". J. Virol. 83 (10): 5109–16. doi:10.1128/JVI.02667-08. PMC 2682064. PMID 19279111.
- Ortín, Juan; Martín-Benito, Jaime (2015). "The RNA synthesis machinery of negative-stranded RNA viruses". Virology. 479–480: 532–544. doi:10.1016/j.virol.2015.03.018. PMID 25824479.
- Schoehn, Guy; Mavrakis, Manos; Albertini, Aurélie; Wade, Richard; Hoenger, Andreas; Ruigrok, Rob W. H. (28 May 2004). "The 12 Å Structure of Trypsin-treated Measles Virus N–RNA". Journal of Molecular Biology. 339 (2): 301–312. doi:10.1016/j.jmb.2004.03.073. PMID 15136034.
- Tawar, Rajiv G.; Duquerroy, Stéphane; Vonrhein, Clemens; Varela, Paloma F.; Damier-Piolle, Laurence; Castagné, Nathalie; MacLellan, Kirsty; Bedouelle, Hugues; Bricogne, Gérard (27 November 2009). "Crystal Structure of a Nucleocapsid-Like Nucleoprotein-RNA Complex of Respiratory Syncytial Virus". Science. 326 (5957): 1279–1283. Bibcode:2009Sci...326.1279T. doi:10.1126/science.1177634. ISSN 0036-8075. PMID 19965480.
- Raymond, Donald D.; Piper, Mary E.; Gerrard, Sonja R.; Smith, Janet L. (29 June 2010). "Structure of the Rift Valley fever virus nucleocapsid protein reveals another architecture for RNA encapsidation". Proceedings of the National Academy of Sciences. 107 (26): 11769–11774. Bibcode:2010PNAS..10711769R. doi:10.1073/pnas.1001760107. ISSN 0027-8424. PMC 2900692. PMID 20547879.
- Hsu, M. T.; Parvin, J. D.; Gupta, S.; Krystal, M.; Palese, P. (1 November 1987). "Genomic RNAs of influenza viruses are held in a circular conformation in virions and in infected cells by a terminal panhandle". Proceedings of the National Academy of Sciences. 84 (22): 8140–8144. Bibcode:1987PNAS...84.8140H. doi:10.1073/pnas.84.22.8140. ISSN 0027-8424. PMC 299494. PMID 2446318.
- Moeller, Arne; Kirchdoerfer, Robert N.; Potter, Clinton S.; Carragher, Bridget; Wilson, Ian A. (21 December 2012). "Organization of the Influenza Virus Replication Machinery". Science. 338 (6114): 1631–1634. Bibcode:2012Sci...338.1631M. doi:10.1126/science.1227270. ISSN 0036-8075. PMC 3578580. PMID 23180774.
- Arranz, Rocío; Coloma, Rocío; Chichón, Francisco Javier; Conesa, Jose Javier; Carrascora, José L.; Valpuesta, José M.; Martín-Benito, Jaime (21 December 2012). "The Structure of Native Influenza Virion Ribonucleoproteins". Science. 338 (6114): 1634–1637. Bibcode:2012Sci...338.1634A. doi:10.1126/science.1228172. PMID 23180776.
- Kranzusch, Philip J.; Whelan, Sean P. J. (1 July 2012). "Architecture and regulation of negative-strand viral enzymatic machinery". RNA Biology. 9 (7): 941–948. doi:10.4161/rna.20345. ISSN 1547-6286. PMC 3495737. PMID 22767259.
- Morin, Benjamin; Kranzusch, Philip J; Rahmeh, Amal A; Whelan, Sean PJ (1 April 2013). "The polymerase of negative-stranded RNA viruses". Current Opinion in Virology. Virus structure and function / Emerging viruses. 3 (2): 103–110. doi:10.1016/j.coviro.2013.03.008. PMC 4159711. PMID 23602472.
- Poch, O; Blumberg, BM; Bougueleret, L; Tordo, N (1990). "Sequence Comparison of Five Polymerases (L proteins) of Unsegmented Negative-strand RNA Viruses: Theoretical Assignment of Functional Domains". Journal of General Virology. 71 (5): 1153–62. doi:10.1099/0022-1317-71-5-1153. PMID 2161049.
- Albertini, Aurélie A. V.; Wernimont, Amy K.; Muziol, Tadeusz; Ravelli, Raimond B. G.; Clapier, Cedric R.; Schoehn, Guy; Weissenhorn, Winfried; Ruigrok, Rob W. H. (21 July 2006). "Crystal Structure of the Rabies Virus Nucleoprotein-RNA Complex". Science. 313 (5785): 360–363. Bibcode:2006Sci...313..360A. doi:10.1126/science.1125280. ISSN 0036-8075. PMID 16778023.
- Green, Todd J.; Zhang, Xin; Wertz, Gail W.; Luo, Ming (21 July 2006). "Structure of the Vesicular Stomatitis Virus Nucleoprotein-RNA Complex". Science. 313 (5785): 357–360. Bibcode:2006Sci...313..357G. doi:10.1126/science.1126953. ISSN 0036-8075. PMID 16778022.
- Ge, Peng; Tsao, Jun; Schein, Stan; Green, Todd J.; Luo, Ming; Zhou, Z. Hong (5 February 2010). "Cryo-EM Model of the Bullet-Shaped Vesicular Stomatitis Virus". Science. 327 (5966): 689–693. Bibcode:2010Sci...327..689G. doi:10.1126/science.1181766. ISSN 0036-8075. PMC 2892700. PMID 20133572.
- Ariza, Antonio; Tanner, Sian J.; Walter, Cheryl T.; Dent, Kyle C.; Shepherd, Dale A.; Wu, Weining; Matthews, Susan V.; Hiscox, Julian A.; Green, Todd J. (1 June 2013). "Nucleocapsid protein structures from orthobunyaviruses reveal insight into ribonucleoprotein architecture and RNA polymerization". Nucleic Acids Research. 41 (11): 5912–5926. doi:10.1093/nar/gkt268. ISSN 0305-1048. PMC 3675483. PMID 23595147.
- "Replication- competent rhabdoviruses with human immunodeficiency virus type 1 coats and green fluorescent protein: entry by a pH-independent pathway". Journal of Virology. 73.
- Calain, Philippe; Curran, Joe; Kolakofsky, Daniel; Roux, Laurent (1 November 1992). "Molecular cloning of natural paramyxovirus copy-back defective interfering RNAs and their expression from DNA". Virology. 191 (1): 62–71. doi:10.1016/0042-6822(92)90166-M. PMID 1329337.
- "Sendai Virus and Simian Virus 5 Block Activation of Interferon-Responsive Genes: Importance for Virus Pathogenesis". Journal of Virology. 73.
- Enami, M.; Luytjes, W.; Krystal, M.; Palese, P. (1 May 1990). "Introduction of site-specific mutations into the genome of influenza virus". Proceedings of the National Academy of Sciences. 87 (10): 3802–3805. Bibcode:1990PNAS...87.3802E. doi:10.1073/pnas.87.10.3802. ISSN 0027-8424. PMC 53991. PMID 2339122.
- Chatterjee, S; Basler, CF; Amarasinghe, GK; Leung, DW (28 August 2016). "Molecular Mechanisms of Innate Immune Inhibition by Non-Segmented Negative-Sense RNA Viruses". Journal of Molecular Biology. 428 (17): 3467–82. doi:10.1016/j.jmb.2016.07.017. PMC 5010489. PMID 27487481.
- Gerlier, Denis; Lyles, Douglas S. (1 September 2011). "Interplay between Innate Immunity and Negative-Strand RNA Viruses: towards a Rational Model". Microbiology and Molecular Biology Reviews. 75 (3): 468–490. doi:10.1128/MMBR.00007-11. ISSN 1092-2172. PMC 3165544. PMID 21885681.
- Leung, Daisy W.; Basler, Christopher F.; Amarasinghe, Gaya K. (2012). "Molecular mechanisms of viral inhibitors of RIG-I-like receptors". Trends in Microbiology. 20 (3): 139–146. doi:10.1016/j.tim.2011.12.005. PMC 3299846. PMID 22325030.
- Yan, Nan; Chen, Zhijian J (2012). "Intrinsic antiviral immunity". Nature Immunology. 13 (3): 214–222. doi:10.1038/ni.2229. PMC 3549670. PMID 22344284.
- O'Shea, John J.; Schwartz, Daniella M.; Villarino, Alejandro V.; Gadina, Massimo; McInnes, Iain B.; Laurence, Arian (14 January 2015). "The JAK-STAT Pathway: Impact on Human Disease and Therapeutic Intervention". Annual Review of Medicine. 66 (1): 311–328. doi:10.1146/annurev-med-051113-024537. ISSN 0066-4219. PMC 5634336. PMID 25587654.
- Rawlings, Jason S.; Rosler, Kristin M.; Harrison, Douglas A. (15 March 2004). "The JAK/STAT signaling pathway". Journal of Cell Science. 117 (8): 1281–1283. doi:10.1242/jcs.00963. ISSN 0021-9533. PMID 15020666.
- Isaacson, Marisa K.; Ploegh, Hidde L. (2009). "Ubiquitination, Ubiquitin-like Modifiers, and Deubiquitination in Viral Infection". Cell Host & Microbe. 5 (6): 559–570. doi:10.1016/j.chom.2009.05.012. PMID 19527883.
- Cadwell, Ken; Coscoy, Laurent (1 July 2005). "Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase". Science. 309 (5731): 127–130. Bibcode:2005Sci...309..127C. doi:10.1126/science.1110340. ISSN 1095-9203. PMID 15994556.
- Neumann, Gabriele; Whitt, Michael A.; Kawaoka, Yoshihiro (1 November 2002). "A decade after the generation of a negative-sense RNA virus from cloned cDNA - what have we learned?". The Journal of General Virology. 83 (Pt 11): 2635–2662. doi:10.1099/0022-1317-83-11-2635. ISSN 0022-1317. PMID 12388800.
- Loney, Colin; Mottet-Osman, Geneviève; Roux, Laurent; Bhella, David (15 August 2009). "Paramyxovirus Ultrastructure and Genome Packaging: Cryo-Electron Tomography of Sendai Virus". Journal of Virology. 83 (16): 8191–8197. doi:10.1128/JVI.00693-09. ISSN 0022-538X. PMC 2715783. PMID 19493999.
- Ruigrok, Rob WH; Crépin, Thibaut; Kolakofsky, Dan (2011). "Nucleoproteins and nucleocapsids of negative-strand RNA viruses". Current Opinion in Microbiology. 14 (4): 504–510. doi:10.1016/j.mib.2011.07.011. PMID 21824806.
- Green, Todd J.; Luo, Ming (14 July 2009). "Structure of the vesicular stomatitis virus nucleocapsid in complex with the nucleocapsid-binding domain of the small polymerase cofactor, P". Proceedings of the National Academy of Sciences. 106 (28): 11713–11718. Bibcode:2009PNAS..10611713G. doi:10.1073/pnas.0903228106. ISSN 0027-8424. PMC 2710649. PMID 19571006.
- Green, Todd J.; Rowse, Michael; Tsao, Jun; Kang, Jungsoon; Ge, Peng; Zhou, Z. Hong; Luo, Ming (15 March 2011). "Access to RNA Encapsidated in the Nucleocapsid of Vesicular Stomatitis Virus". Journal of Virology. 85 (6): 2714–2722. doi:10.1128/JVI.01927-10. ISSN 0022-538X. PMC 3067934. PMID 21177817.
- Luo, Ming; Green, Todd J.; Zhang, Xin; Tsao, Jun; Qiu, Shihong (1 January 2007). "Structural comparisons of the nucleoprotein from three negative strand RNA virus families". Virology Journal. 4: 72. doi:10.1186/1743-422X-4-72. ISSN 1743-422X. PMC 2031895. PMID 17623082.
- Green, TJ; Cox, R; Tsao, J; Rowse, M; Qiu, S; Luo, M (April 2014). "Common mechanism for RNA encapsidation by negative-strand RNA viruses". Journal of Virology. 88 (7): 3766–75. doi:10.1128/jvi.03483-13. PMC 3993539. PMID 24429372.
- Ortín, J; Martín-Benito, J (May 2015). "The RNA synthesis machinery of negative-stranded RNA viruses". Virology. 479–480: 532–44. doi:10.1016/j.virol.2015.03.018. PMID 25824479.
- Neumann, G; Whitt, MA; Kawaoka, Y (November 2002). "A decade after the generation of a negative-sense RNA virus from cloned cDNA - what have we learned?". The Journal of General Virology. 83 (Pt 11): 2635–62. doi:10.1099/0022-1317-83-11-2635. PMID 12388800.
- Viswanathan, K; Früh, K; DeFilippis, V (August 2010). "Viral hijacking of the host ubiquitin system to evade interferon responses". Current Opinion in Microbiology. 13 (4): 517–23. doi:10.1016/j.mib.2010.05.012. PMC 2939720. PMID 20699190.