Nasal cycle
The nasal cycle is the often unnoticed[1][2] alternating partial congestion and decongestion of the nasal cavities in humans and other animals. It is a physiological congestion of the nasal conchae, also called the nasal turbinates, due to selective activation of one half of the autonomic nervous system by the hypothalamus. It should not be confused with pathological nasal congestion. The nasal cycle was studied and discussed in the ancient yoga literature of pranayama.[3] In the modern western literature, it was first described by the German physician Richard Kayser in 1895.[4]
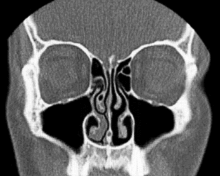
Description
In 1927, Heetderks[5] described the alternating turgescence of the inferior turbinates in 80% of a normal population. According to Heetderks, the cycle is the result of alternating congestion and decongestion of the nasal conchae or turbinates, predominantly the inferior turbinates, which are by far the largest of the turbinates in each nasal fossa. Turbinates consist of bony projections covered by erectile tissue, much like the tissues of the penis and clitoris. The turbinates in one fossa fill up with blood while the opposite turbinates decongest by shunting blood away. This cycle, which is controlled by the autonomic nervous system, has a mean duration of two and a half hours. He further observed and documented that the turbinates in the dependent nasal fossa fill when the patient is in the lateral decubitus (lying down on your side) position. Some postulate that this alternating positional obstruction has the purpose of causing a person to turn from one side to the other while sleeping in order to prevent bedsores. Others note that the asymmetric airflow may have some benefit to overall olfactory sensitivity.[6] The nasal cycle is an alternation in both time and between left and right sides, with the total resistance in the nose remaining constant. In patients with a fixed septal deviation and intermittent nasal obstruction, the interplay of the nasal cycle becomes evident; the sensation of obstruction frequently mirrors the congestion phase.[6]
It is possible that the nasal cycle may exacerbate the nasal congestion caused by the common cold, as the lack of motility of the cilia in one half of the nose may lead to an uncomfortable sensation of not being able to shift mucus by blowing the nose.
Benefits in breathing
It has been shown that the cilia of the congested side suspend their motility until that side decongests. Thus the cycle ensures that one side of the nose is always moist, to facilitate humidification, which is one of the three functions of the nose, the other two being filtration and warming of inspired air prior to its entering the lungs.[6]
Benefits in olfaction
Some odor chemicals bind with olfactory receptors easily, even under conditions of high airflow, and other odors need more time, under low airflow conditions, to bind with receptors. With high airflow on one side and low airflow on the other side, the olfactory center detects a greater range of smells.[7][8][9]
Distinction
The nasal cycle should not be confused with pathological nasal congestion: individuals with normal nasal breathing usually do not realize their breathing is asymmetric unless there is underlying nasal obstruction.[1] In pathological conditions, however, the nasal cycle may influence the symptoms.[2]
Research on the effects
In 1994, breathing through alternate nostrils showed effects on brain hemisphere symmetry on EEG topography.[10] D.S. Shannahoff-Khalsa published in 2007 on the effect of this cycle and manipulation through forced nostril breathing on one side on the endogenous ultradian rhythms of the autonomic and central nervous system.[11]
More recent research has shown no statistically significant correlation between spontaneously (i.e. not forced) dominant nostril and active brain hemisphere.[12]
References
- Josephson, J. S. (2006). Sinus Relief Now: The Ground-Breaking 5-Step Program for Sinus, Allergy, And Asthma Sufferers. Penguin Group. p. 15. ISBN 978-0-39953-298-6.
- Huizing, E. H.; de Groot, J. A. M. (2003). Functional Reconstructive Nasal Surgery. Thieme. p. 52. ISBN 978-1-58890-081-4.
- Swami Muktibodhananda (2004). "Prana: Vital Energy". Swara yoga : the tantric science of brain breathing. Munger, Bihar, India: Yoga Publications Trust. p. 12. ISBN 978-81-85787-36-7.
- Richard Kayser: Die exakte Messung der Luftdurchgängigkeit der Nase. Arch. Laryng. Rhinol. (Berl.) 8, 101 (1895)
- Cummings: Otolaryngology: Head & Neck Surgery, 4th ed
- Thomas Hummel; Antje Welge-Lüssen (1 January 2006), Taste and Smell: An Update, Karger Medical and Scientific Publishers, pp. 12–, ISBN 978-3-8055-8123-3
- Sobel, Noam; Khan, Rehan M.; Saltman, Amnon; Sullivan, Edith V.; Gabrieli, John D. E. (1999-11-04). "Olfaction: The world smells different to each nostril". Nature. Macmillan Magazines Ltd. 402 (6757): 35. Bibcode:1999Natur.402...35S. doi:10.1038/46944. ISSN 0028-0836. PMID 10573415.
- O'Toole, Kathleen (1999-11-10). "Smelling roses: Your nostrils may part ways". Retrieved 2015-06-26.
- Kruszelnicki, Karl (2000-11-01). "Nostrils smell differently 1". Retrieved 2015-06-26.
- Stancák A Jr, Kuna M (October 1994). "EEG changes during forced alternate nostril breathing". Int J Psychophysiol. 18 (1): 75–9. doi:10.1016/0167-8760(84)90017-5. PMID 7876041.CS1 maint: uses authors parameter (link)
- D.S. Shannahoff-Khalsa: Selective Unilateral Autonomic Activation: Implications for Psychiatry. CNS Spectr. 2007, 12(8):625-634
- Samantaray, S; Telles, S (2008). "Nostril dominance at rest associated with performance of a left hemisphere-specific cancellation task". International Journal of Yoga. 1 (2): 56–59. doi:10.4103/0973-6131.43542. PMC 3144611. PMID 21829285.