Mesencephalic locomotor region
The mesencephalic locomotor region (MLR) is a functionally defined area of the midbrain that is associated with the initiation and control of locomotor movements in vertebrate species.[1][2]
| Mesencephalic locomotor region | |
|---|---|
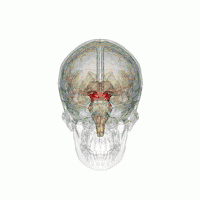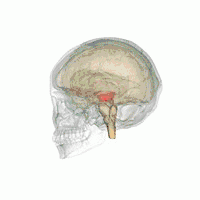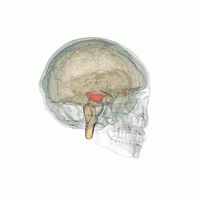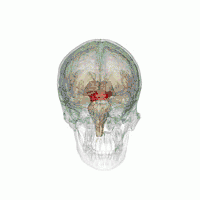 The midbrain. | |
| Details | |
| Part of | Brainstem |
| Identifiers | |
| Acronym(s) | MLR |
| Anatomical terminology | |
Neuroanatomical organization
The MLR was first described by Shik and colleagues in 1966 when they observed that electrical stimulation of a region of the midbrain in decerebrate cats produced walking and running behavior on a treadmill.[3] Twenty-eight years later, Masdeu and colleagues described the presence of a MLR in humans.[4] It is now widely acknowledged that, along with other motor control centers of the brain, the MLR plays an active role in initiating and modulating the spinal neural circuitry to control posture and gait.[5] Anatomically, as the name suggests, the MLR is located in the mesencephalon (or midbrain), ventral to the inferior colliculus and near the cuneiform nucleus.[6] Although identifying the exact anatomical substrates of the MLR has been subject to considerable debate, the pedunculopontine nucleus (PPN), lateral cuneiform nucleus, and midbrain extrapyramidal area are thought to form the neuroanatomical basis of the MLR.[7][8] Nuclei within the MLR receive inputs from the substantia nigra of the basal ganglia and neural centers within the limbic system.[9] Projections from the MLR descend via the medullary and pontine reticulospinal tracts to act on spinal motor neurons supplying the trunk and proximal limb flexors and extensors.[2][5][10]
The PPN within the MLR is composed of a diverse population of neurons containing the neurotransmitters gamma-amino-butyric acid (GABA), glutamate, and acetylcholine (ACh).[11] Results from animal and clinical studies suggest that cholinergic neurons in the PPN play a crucial role in modulating both the rhythm of locomotion and postural muscle tone.[12][13] Glutamatergic and cholinergic inputs from the MLR may be responsible for regulating the excitability of reticulospinal neurons that in turn project to spinal central pattern generators to initiate stepping.[1][14]
Clinical significance
The integration of motor and sensory information during walking involves communication between cortical, subcortical, and spinal circuits. Step-like motor patterns of the lower extremities can be induced through activation of the spinal circuitry alone;[15] however, supraspinal input is necessary for functional bipedal walking in humans.[16][17] Pathologies of the nuclei within the MLR have been associated with a combination of clinical features that are unique to midbrain dysfunction and can be differentiated from other subcortical neurological conditions such as those associated with Parkinsonism and cerebellar lesions.[18]
In a clinical case series, three adult males with isolated lesions of the MLR presented with gait hesitation and gait ataxia characterized by stepping that lacked uniform direction, amplitude, and rhythmicity.[19] Although gait hesitation and ataxia are also clinical features of Parkinson's disease and lesions of the cerebellum, respectively, the authors noted that the patients did not display any other common signs or symptoms associated with these neurological conditions, suggesting that pathologies of the midbrain can produce gait disturbances even when cerebellar and basal ganglia function are intact. In a study investigating high-level gait and balance disorders in elderly adults who had no evidence of rheumatologic, orthopedic, or neurologic disease, brain imaging data revealed an association between reduced gray matter density of the PPN and cuneiform nucleus and impaired gait initiation, step execution, and postural control.[20] Additionally, among eighteen individuals with Parkinson's disease who either did or did not experience Freezing of Gait, functional magnetic resonance imaging revealed reduced activity in the MLR and supplementary motor area among those individuals who experienced episodic gait hesitation.[21] Freezing of Gait has also been associated with functional reorganization of supraspinal locomotor networks whereby altered connectivity and communication between the supplementary motor area and MLR were observed.[22] These findings suggest that the MLR does in fact play a unique role in human locomotion, especially with respect to step initiation and motor planning.
Deep brain stimulation
Given the role of the MLR in gait initiation and postural control, researchers and clinicians have investigated the effects of targeted deep brain stimulation (DBS) on gait disturbances in clinical populations.[23][24] Plaha and Gill reported significant improvements in gait dysfunction and postural instability in two patients with advanced Parkinson's disease who were treated using DBS electrodes implanted in the region of the PPN.[25] Likewise, in a more recent study, six patients with Parkinson's disease demonstrated improvements in posture, gait, and postural stability following 6 months of DBS to the PPN and subthalamic nucleus.[26] Bachmann and colleagues applied DBS to the MLR in rats with chronic, incomplete spinal cord injury and reported improved hindlimb function and near normal restoration of locomotor function following treatment.[27]
See also
References
- Le Ray, D; Juvin, L; Ryczko, D; Dubuc, R (2011). "Chapter 4 - Supraspinal control of locomotion: the mesencephalic locomotor region". Progress in Brain Research. 188: 51–70.
- Pahapill, P; Lozano, A (2000). "The pedunculopontine nucleus and Parkinson's disease". Brain. 123: 1767–1783. doi:10.1093/brain/123.9.1767.
- Shik, ML; Severin, FV; Orlofsky, GN (1966). "Control of walking and running by means of electrical stimulation of the midbrain". Biophysics (Oxf). 11: 756–765.
- Masdeu, JC; Alampur, U; Cavaliere, R; Tavoulareas, G (1994). "Astasia and gait failure with damage of the pontomesencephalic locomotor region". Annals of Neurology. 35: 619–621. doi:10.1002/ana.410350517.
- Takakusaki, K (2017). "Functional neuroanatomy for posture and gait control". Journal of Movement Disorders. 10 (1): 1–17. doi:10.14802/jmd.16062.
- Pearson, KG; Gordon, JE (2013). Principles of Neural Science: Locomotion (5th ed.). New York: The McGraw-Hill Companies, Inc.
- Skinner, RD; Garcia-Rill, E (1984). "The mesencephalic locomotor region (MLR) in the rat". Brain Research. 323: 385–389. doi:10.1016/0006-8993(84)90319-6.
- Sherman, D; Fuller, PM; Marcus, J; Yu, J; Zhang, P; Chamberlin, NL; Saper, CB; Lu, J (2015). "Anatomical location of the mesencephalic locomotor region and its possible role in locomotion, posture, cataplexy, and Parkinsonism". Frontiers in Neurology. 6 (140): 1–13. doi:10.3389/fneur.2015.00140.
- Sherman, D; Fuller, PM; Marcus, J; Yu, J; Zhang, P; Chamberlin, N; Saper, C; Lu, J (2015). "Anatomical location of the mesencephalic locomotor region and its possible role in locomotion, posture, cataplexy, and Parkinsonism". Frontiers in Neurology. 6 (140): 1–13. doi:10.3389/fneur.2015.00140.
- Takakusaki, K; Tomita, N; Yano, M (2008). "Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction". Journal of Neurology. 255 (Suppl 4): 19–29.
- Takakusaki, K; Chiba, R; Tsukasa, N; Okumura, T (2016). "Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems". Journal of Neural Transmission. 123: 695–729. doi:10.1007/s00702-015-1475-4.
- Bohnen, NI; Albin, RL (2011). "The cholinergic system and Parkinson disease". Behavioural Brain Research. 221: 564–573. doi:10.1016/j.bbr.2009.12.048.
- Takakusaki, K; Obara, K; Nozu, T; Okumura, T (2011). "Modulatory effects of the GABAergic basal ganglia neurons on the PPN and the muscle tone inhibitory system in cats". Archives Italiennes de Biologie. 149: 385–405.
- Skinner, RD; Kinjo, N; Henderson, V; Garcia-Rill, E (1990). "Locomotor projections from the pedunculopontine nucleus to the spinal cord". Neurological Reports. 1: 183–186. doi:10.1097/00001756-199011000-00001.
- Whelan, PJ (2003). "Developmental aspects of spinal locomotor function: insights from using the in vitro mouse spinal cord preparation". Journal of Physiology. 553 (Pt 3): 695–706. doi:10.1113/jphysiol.2003.046219.
- Nielsen, Jens Bo (2003). "How we walk: central control of muscle activity during human walking". Neuroscientist. 9 (3): 195–204. doi:10.1177/1073858403009003012.
- Capaday, Charles (2002). "The special nature of human walking and its neural control". Trends in Neurosciences. 25 (7): 370–376. doi:10.1016/s0166-2236(02)02173-2.
- Ruchalski, K; Hathout, GM (2012). "A medley of midbrain maladies: a brief review of midbrain anatomy and syndromology for radiologists". Radiology Research and Practice. 2012: 1–11. doi:10.1155/2012/258524.
- Hathout, GM; Bhidayasiri, R (2005). "Midbrain ataxia: an introduction to the mesencephalic locomotor region and the pedunculopontine nucleus". American Journal of Roentgenology. 184: 953–956. doi:10.2214/ajr.184.3.01840953.
- Demain, A; Westby, M; Fernandez-Vidal, S; Karachi, C; Bonneville, F; Do, MC; Delmaire, C; Dormont, D; Bardinet, E; Agid, Y; Chastan, N; Welter, ML (2014). "High-level gait and balance disorders in the elderly: a midbrain disease?". Journal of Neurology. 261: 196–206. doi:10.1007/s00415-013-7174-x.
- Peterson, DS; Pickett, KA; Duncan, R; Perlmutter, J; Earhart, GM (2014). "Gait-related brain activity in people with Parkinson disease with freezing of gait". PLoS One. 9 (3): e90634. doi:10.1371/journal.pone.0090634.
- Fling, BW; Cohen, RG; Mancini, M; Carpenter, SD; Fair, DA; Nutt, JG; Horak, FB (2014). "Functional reorganization of the locomotor network in Parkinson patients with freezing gait". PLoS One. 9 (6): e100291. doi:10.1371/journal.pone.0100291.
- Hamani, C; Scellig, S; Laxton, A; Lozano, AM (2007). "The pedunculopontine nucleus and movement disorders: anatomy and the role for deep brain stimulation". Parkinsonism and Related Disorders. 13: S276-S280. doi:10.1016/s1353-8020(08)70016-6.
- Richardson, M (2014). "Deep brain stimulation for locomotor recovery following spinal cord injury". Science Times. 74 (2): N18-N19. doi:10.1227/01.neu.0000442979.07078.ac.
- Plaha, P; Gill, S (2005). "Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease". NeuroReport. 16 (17): 1883–1887. doi:10.1097/01.wnr.0000187637.20771.a0.
- Stefani, A; Lozano, AM; Peppe, A; Stanzione, P; Galati, S; Tropepi, D (2007). "Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease". Brain. 130: 1596–1607. doi:10.1093/brain/awl346.
- Bachmann, LC; Matis, A; Lindau, NT; Felder, P; Gullo, M; Schwab, ME (2013). "Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats". Science Translational Medicine. 5 (208): 208.