Merkel-cell carcinoma
Merkel-cell carcinoma (MCC) is a rare and aggressive skin cancer occurring in about 3 per 1,000,000 population.[1] It is also known as cutaneous APUDoma, primary neuroendocrine carcinoma of the skin, primary small cell carcinoma of the skin, and trabecular carcinoma of the skin.[2] Factors involved in the development of MCC include the Merkel cell polyomavirus (MCPyV or MCV), a weakened immune system, and exposure to ultraviolet radiation.[3][4] Merkel-cell carcinoma usually arises on the head, neck, and extremities, as well as on the perianal and eyelid.[5] It is more common in elderly people, people who have a weakened immune system, and people who were exposed to UV radiation. MCC is less common in children.[1][5]
| Merkel-cell carcinoma | |
|---|---|
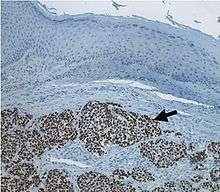
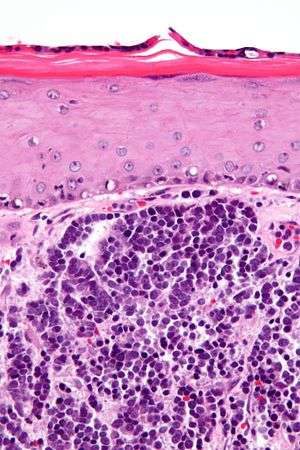 | |
| Micrograph of a Merkel-cell carcinoma. H&E stain. | |
| Specialty | Oncology |
Signs and symptoms

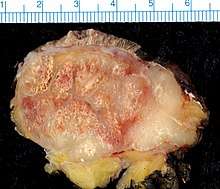
Merkel-cell carcinoma (MCC) usually presents as a firm, painless, nodule (up to 2 cm diameter) or mass (>2 cm diameter). These flesh-colored, red, or blue tumors typically vary in size from 0.5 cm (less than one-quarter of an inch) to more than 5 cm (2 inches) in diameter, and usually enlarge rapidly. Although MCC's may arise almost anywhere on the body, about half originate on sun-exposed areas of the head and neck, one-third on the legs, and about one-sixth on the arms. In about 12% of cases, no obvious anatomical site of origin ("primary site") can be identified.[6] The most significant clues in the diagnosis of MCC were summarized 2008 in the acronym AEIOU (Asymptomatic/lack of tenderness, Expanding rapidly, Immune suppression, Older than 50 years, and Ultraviolet-exposed site on a person with fair skin).[7] Ninety percent of MCC's have 3 or more of those features.[8] MCC is sometimes mistaken for other histological types of cancer, including basal cell carcinoma, squamous cell carcinoma, malignant melanoma, lymphoma, and small cell carcinoma, or as a benign cyst.[9] Merkel cell carcinomas have been described in children, however pediatric cases are very rare.[10]
Merkel-cell cancers tend to invade locally, infiltrating the underlying subcutaneous fat, fascia, and muscle, and typically metastasize early in their natural history, most often to the regional lymph nodes. MCCs also spread aggressively through the blood vessels to many organs, particularly to liver, lung, brain, and bone.[11]
Pathophysiology
Several factors are involved in the pathophysiology of MCC, including a virus called Merkel cell polyomavirus (MCV), ultraviolet radiation (UV) exposure, and weakened immune function.[12]
Merkel cell polyomavirus
MCV likely contributes to the development of the majority of MCC.[13] About 80% of MCC tumors are infected with MCV, with the virus integrated in a monoclonal pattern,[13] indicating that the infection was present in a precursor cell before it became cancerous. MCV, a polyomavirus, is often present in MCC but no clear evidence suggests that the relationship is causal.[3] MCV is ubiquitous and is thought to be part of the human skin microbiome.[14] Intriguingly, most MCV viruses obtained so far from tumors have specific mutations that render the virus uninfectious.[15][16] People with MCC whose tumors contain MCV have higher antibody levels against the virus than similarly infected healthy adults.[17] A study of a large patient registry from Finland suggests that individuals with MCV-positive MCC's have better prognoses than do people with MCC who do not have a MCV infection.[18] Like other tumor viruses, most people who are infected with MCV do not develop MCC. As of 2008, it was unknown what other steps or co-factors were required for MCC-type cancers to develop.[19]
UV light
At least 20% of MCC tumors are not infected with MCV, suggesting that MCC may have other causes, especially sunlight or ultraviolet light as in a tanning beds. MCC can also occur together with other sun exposure-related skin cancers that are not infected with MCV (i.e. basal cell carcinoma, squamous cell carcinoma, melanoma). Ultraviolet radiation such as in sun exposure increases the risk in MCC development, consistent with the fact that MCCs occur more commonly in sun-exposed areas.[7][9]
Immunosuppression
The incidence of MCC is increased in conditions with defective immune functions such as malignancy, HIV infection, and organ transplant patients, etc.[20] Mutations in MCC occur more frequently than would otherwise be expected among people who are immunosuppressed, such as people who have undergone an organ transplant, people with AIDS, and elderly people, suggesting that the initiation and progression of the disease is modulated by the immune system.[7] While infection with MCV is common in humans,[21] In addition, a high incidence of this tumor has been observed in people who are effected by autoimmune disease who are being treated with immunosuppressants, such as TNF inhibitors.[12]
Diagnosis
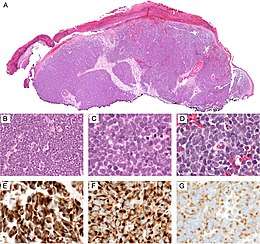
Diagnosis of MCC begins with a clinical examination of the skin and lymph nodes.[23] Following, definitive diagnosis of Merkel cell carcinoma (MCC) requires examination of biopsy tissue to identify its histopathologic features.[23][24] An ideal biopsy specimen is either a punch biopsy or a full-thickness incisional biopsy of the skin including full-thickness dermis and subcutaneous fat. On light microscopy, MCC shows basaloid tumor nests with neuroendocrine features ("salt and pepper" chromatin, scarce cytoplasm, and brisk mitotic activity).[23][24] In addition to standard examination under light microscopy, immunohistochemistry (IHC) is also generally required to differentiate MCC from other morphologically similar tumors such as small cell lung cancer, the small cell variant of melanoma, various cutaneous leukemic/lymphoid neoplasms, and Ewing's sarcoma. Similarly, most experts recommend longitudinal imaging of the chest, typically a CT scan, to rule out that the possibility that the skin lesion is a skin metastasis of an underlying small cell carcinoma of the lung. Once an MCC diagnosis is made, a sentinel lymph node biopsy is recommended as a part of the staging work-up needed to determine prognosis and subsequent treatment options.[23][24]
Prevention
Sunlight exposure is thought to be one of the causes of Merkel cell carcinoma (MCC). The World Health Organization, American Academy of Dermatology, and Skin Cancer Foundation recommend the following measures to prevent excessive UV exposure and skin cancer:[25][26][27]
- Limiting sun exposure between the hours of 10am and 4pm, when UV rays are the strongest
- Seeking shade when UV rays are most intense
- Wearing sun-protective clothing including a wide brim hat, sunglasses, and tightly-woven, loose-fitting clothing
- Using sunscreen
- Avoiding tanning beds and artificial UV exposure
Treatment
Early diagnosis and treatment of Merkel-cell cancers are important factors in decreasing the chance of metastasis, after which it is exceptionally difficult to cure.
Surgery
The goal of surgical excision is to remove the MCC with negative resection margin, meaning the tumour has been excised completely with a surrounding border of healthy tissue.[28] Complete excision is associated with significantly higher survival rates.[28] Surgical margins should extend 1 to 2 cm beyond the border of the MCC and may include underlaying fascia and muscle if the MCC has invaded deeper than the dermis.[28] Surgical excision should be coordinated with the sentinel lymph node biopsy (SNLB).[28] Reconstruction may also be needed if the surgical treatment requires extensive tissue removal.[28] However, reconstruction should not occur until negative margins and a negative SLNB have been confirmed.[28]
Radiation and Chemotherapy
Evidence on the efficacy of post-operative radiation therapy (RT) for Merkel cell carcinoma is inconclusive as of 2018.[28] Therefore it is unclear which people with MCC should be receiving post-operative RT.[28] However, it has been recommended that observation is sufficient for people will small primary lesions (<1 cm) that have been excised with clear margins and no lymph node involvement, or immunosuppression.[28] Post-operative RT, which is given after surgical treatment, is recommended for cases with larger primary lesions, with positive margins indicating the possibility of an incomplete excision, and other risk factors for reoccurrence including positive lymph node involvement and immunosuppression.[28] If the person is to receive RT, the delay between the surgical treatment and RT should be minimized to optimize the clinical outcome.[28] Improved clinical outcomes include better wound healing, reduced recurrence rates, and increase overall survival.[28]
Chemotherapy may be used to treat both primary and metastatic MCC.[29] Although the definitive role of chemotherapy is unknown chemotherapy plays a role in the treatment, especially in MCC of head and neck regions.[29]
Sentinel lymph node biopsy
Sentinel lymph node biopsy (SLNB) detects MCC spread in one third of people with MCC whose tumors would have otherwise been clinically and radiologically understaged, and who may not have received treatment to the involved node bed. There was a significant benefit of adjuvant nodal therapy, but only when the SLNB was positive. Thus, SLNB is important for both prognosis and therapy and should be performed routinely for patients with MCC. In contrast, computed tomographic scans have poor sensitivity in detecting nodal disease as well as poor specificity in detecting distant disease.[30]
Drug therapy
Immunotherapies, namely inhibitors of the PD1-PDL1 checkpoint signalling pathway, are novel anticancer agents that have shown benefit in advanced-stage MCC or chemotherapy-resistant MCC.[31][32][33] These checkpoint inhibitors reactivate the immune response, enabling the immune system to target cancer cells for destruction.[34] In March 2017, the U.S. Food and Drug Administration granted accelerated approval to avelumab, a PDL1 inhibitor, to treat adults and children above 12 years with metastatic MCC. Avelumab, a checkpoint-inhibitor targets the PD-1/PD-L1 pathway (proteins found on the body's immune cells and some cancer cells) to help the body's immune system attack cancer cells.[35] In December 2018, the U.S. Food and Drug Administration granted accelerated approval to pembrolizumab(KEYTRUDA®, Merck & Co. Inc.) for all ages (adults and pediatrics) with recurrent locally advanced or metastatic Merkel cell carcinoma. Keytruda (pembrolizumab), is another checkpoint-inhibitor targeting the PD-1/PD-L1 pathway.[36] Other than immunotherapies, there has been hope that new targeted anticancer therapy for people with distant and systemic MCC disease would be available in the near future, by targeting the MCV either to prevent infection or to inhibit viral-induced carcinogenesis.[37]
National Comprehensive Cancer Network guidelines recommend PD-1 inhibitors, either nivolumab, pembrolizumab or avelumab, for people with disseminated MCC; systemic therapy is not recommended for early stage MCC.[8]
Prognosis
According to the American Joint Committee on Cancer (AJCC), the natural course of MCC is “variable and depends heavily on the stage at diagnosis".[38] Staging of MCC is classified according to the TNM staging system, a notation system that describes the stage of cancer according to the size of the primary tumor (T), the degree of spread to regional lymph nodes (N), and the presence of distant metastasis (M).[38] A combination of T, N, and M stages dictate the final clinical stage group (0, I, IIA, IIB, IIIA, IIIB, IV).[39] Advanced stage (i.e. increased size of the tumor, spreading of the tumor into surrounding and/or distant tissue, and involvement of lymph nodes) is associated with lower survival rates.[40]
The National Cancer Data Base has survival rates collected from nearly 3000 MCC patients from year 1996-2000 with 5-year survival rates listed as follows:[41] Stage IA: 80%. Stage IB: 60%. Stage IIA: 60%. Stage IIB: 50%. Stage IIC: 50%. Stage IIIA: 45%. Stage IIIB: 25%. Stage IV: 20%. 5 yr survival may be 51% among people with localized disease, 35% for those with nodal disease, and 14% with metastases to a distant site.[8]
Several other features may also affect prognosis, independent of tumor stage. They include MCV viral status, histological features, and immune status. In viral status, MCV large tumor antigen (LT antigen) and retinoblastoma protein (RB protein) expression correlates with more favorable prognosis, while p63 expression correlates with a poorer prognosis.[42][43] Histological features such as intratumoral CD8+ T lymphocyte infiltration may be associated with a favorable prognosis, while lymphovascular infiltrative pattern may be associated with a poorer prognosis.[44][45] Immune status, especially T cell immunosuppression (e.g., organ transplant, HIV infection, certain malignancy) predicts poorer prognosis and higher mortality.[46]
The antibody titer in the blood to the Merkel cell polyomavirus oncoprotein can be used as a treatment response biomarker in people that have detectable antibodies at the time of diagnosis.[47][48]
Epidemiology
This skin cancer occurs most often in Caucasians between 60 and 80 years of age, and its rate of incidence is about twice as high in males as in females. MCC is not a very common skin cancer. In 2013, the annual incidence rate was around 0.7 per 100,000 persons in the U.S.[49] As of 2005, roughly 2,500 new cases of MCC have been diagnosed each year in the United States,[49] as compared to around 60,000 new cases of malignant melanoma and over 1 million new cases of nonmelanoma skin cancer.[50] Similar to melanoma, the incidence of MCC in the US is increasing rapidly.[9]
Since 2006, it has been known that other primary cancers increase the risk of MCC significantly, especially in those with the prior multiple myeloma, chronic lymphocytic leukemia, and malignant melanoma.[51]
Immunosuppression can profoundly increase the odds of developing MCC.[12] As of 2013, MCC occurred 30 times more often in people with chronic lymphocytic leukemia and 13.4 times more often in people with advanced HIV as compared to the general population; solid organ transplant recipients had a 10-fold increased risk compared to the general population.[52] A 2015 review of transplant recipients showed an up to 24-fold increased risk of MCC compared to the general population.[53] In addition a high incidence of this tumor has been observed in autoimmune disease affected patients treated with immunosuppressants, such as TNF inhibitors.[12]
Society and culture
- Avigdor Arikha – Paris-based painter and art historian
- David Brudnoy – Boston talk radio host
- Al Copeland – New Orleans entrepreneur, powerboat racer
- Al Davis – Principal owner of the Oakland Raiders of the National Football League
- Ed Derwinski – U.S. Representative from Illinois and 1st Secretary of Veterans Affairs
- Leonard Hirshan – Showbusiness agent and manager.
- Max Perutz – Nobel Prize–winning chemist
- Lindsay Thompson – Former Premier of Victoria, Australia
- Joe Zawinul – Jazz-fusion keyboardist and composer [54]
- John Fitch – Race car driver and road safety pioneer
- Carl Mundy – 30th Commandant of the United States Marine Corps
- Geoffrey Penwill Parsons – Pianist
- Maria Bueno - Tennis player[55]
References
- "Merkel-cell carcinima". Dynamed. Retrieved 2019-11-11.
- Dermatology. Bolognia, Jean., Jorizzo, Joseph L., Rapini, Ronald P. (2nd ed.). [St. Louis, Mo.]: Mosby/Elsevier. 2008. ISBN 978-1-4160-2999-1. OCLC 212399895.CS1 maint: others (link)
- Kervarrec, Thibault; Samimi, Mahtab; Guyétant, Serge; Sarma, Bhavishya; Chéret, Jérémy; Blanchard, Emmanuelle; Berthon, Patricia; Schrama, David; Houben, Roland; Touzé, Antoine (2019-06-10). "Histogenesis of Merkel Cell Carcinoma: A Comprehensive Review". Frontiers in Oncology. 9: 451. doi:10.3389/fonc.2019.00451. ISSN 2234-943X. PMC 6579919. PMID 31245285.
- Rotondo, John Charles; Bononi, Ilaria; Puozzo, Andrea; Govoni, Marcello; Foschi, Valentina; Lanza, Giovanni; Gafà, Roberta; Gaboriaud, Pauline; Touzé, Françoise Antoine; Selvatici, Rita; Martini, Fernanda (2017-07-15). "Merkel Cell Carcinomas Arising in Autoimmune Disease Affected Patients Treated with Biologic Drugs, Including Anti-TNF". Clinical Cancer Research. 23 (14): 3929–3934. doi:10.1158/1078-0432.CCR-16-2899. ISSN 1078-0432. PMID 28174236.
- Patterson, James W. (James Willis), 1946- (2014-12-07). Weedon's skin pathology. Hosler, Gregory A. (Fourth ed.). [Edinburgh?]. ISBN 978-0-7020-6200-1. OCLC 900724639.CS1 maint: multiple names: authors list (link)
- Deneve JL, Messina JL, Marzban SS et al. Merkel Cell Carcinoma of Unknown Primary Origin. Ann Surg Oncol 2012 Jan 21.
- Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Peñas PF, Nghiem P (March 2008). "Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features". Journal of the American Academy of Dermatology. 58 (3): 375–81. doi:10.1016/j.jaad.2007.11.020. PMC 2335370. PMID 18280333.
- Voelker, Rebecca (2018). "Why Merkel Cell Cancer is Garnering More Attention". JAMA. 320 (1): 18–20. doi:10.1001/jama.2018.7042. PMID 29898204.
- Schrama D, Ugurel S, Becker JC (March 2012). "Merkel cell carcinoma: recent insights and new treatment options". Current Opinion in Oncology. 24 (2): 141–9. doi:10.1097/CCO.0b013e32834fc9fe. PMID 22234254.
- Paulson KG, Nghiem P (June 2019). "One in a hundred million: Merkel cell carcinoma in pediatric and young adult patients is rare but more likely to present at advanced stages based on US registry data". Journal of the American Academy of Dermatology. 80 (6): 1758–1760. doi:10.1016/j.jaad.2018.08.021. PMC 6487227. PMID 30165170.
- "Merkel Cell Carcinoma Treatment". National Cancer Institute. 2006-02-21. Retrieved 2018-03-04.
- Rotondo JC, Bononi I, Puozzo A, Govoni M, Foschi V, Lanza G, et al. (July 2017). "Merkel Cell Carcinomas Arising in Autoimmune Disease Affected Patients Treated with Biologic Drugs, Including Anti-TNF". Clinical Cancer Research. 23 (14): 3929–3934. doi:10.1158/1078-0432.CCR-16-2899. PMID 28174236.
- Amber K, McLeod MP, Nouri K (February 2013). "The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma". Dermatologic Surgery. 39 (2): 232–8. doi:10.1111/dsu.12079. PMID 23387356.
- Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB (June 2010). "Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin". Cell Host & Microbe. 7 (6): 509–15. doi:10.1016/j.chom.2010.05.006. PMC 2919322. PMID 20542254.
- Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, Chang Y (October 2008). "T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus". Proceedings of the National Academy of Sciences of the United States of America. 105 (42): 16272–7. Bibcode:2008PNAS..10516272S. doi:10.1073/pnas.0806526105. PMC 2551627. PMID 18812503.
- Sastre-Garau X, Peter M, Avril MF, Laude H, Couturier J, Rozenberg F, et al. (May 2009). "Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis". The Journal of Pathology. 218 (1): 48–56. doi:10.1002/path.2532. PMID 19291712.
- Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, Moschos S, et al. (September 2009). "Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays". International Journal of Cancer. 125 (6): 1250–6. doi:10.1002/ijc.24509. PMC 2747737. PMID 19499548.
- Sihto H, Kukko H, Koljonen V, Sankila R, Böhling T, Joensuu H (July 2009). "Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma". Journal of the National Cancer Institute. 101 (13): 938–45. doi:10.1093/jnci/djp139. PMID 19535775.
- "New virus linked to rare but lethal skin cancer". The Age. Archived from the original on 2008-01-19. Retrieved 2008-02-26.
- Tello TL, Coggshall K, Yom SS, Yu SS (March 2018). "Merkel cell carcinoma: An update and review: Current and future therapy". Journal of the American Academy of Dermatology. 78 (3): 445–454. doi:10.1016/j.jaad.2017.12.004. PMID 29229573.
- Kean JM, Rao S, Wang M, Garcea RL (March 2009). "Seroepidemiology of human polyomaviruses". PLoS Pathogens. 5 (3): e1000363. doi:10.1371/journal.ppat.1000363. PMC 2655709. PMID 19325891.
- Originally copied from: Nguyen AH, Tahseen AI, Vaudreuil AM, Caponetti GC, Huerter CJ (2017). "Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review". Gynecologic Oncology Research and Practice. 4 (1): 2. doi:10.1186/s40661-017-0037-x. PMC 5264489. PMID 28138393. - "Fig 2- available via license: Creative Commons Attribution 4.0 International"
- Amaral, Teresa; Leiter, Ulrike; Garbe, Claus (2017-12-01). "Merkel cell carcinoma: Epidemiology, pathogenesis, diagnosis and therapy". Reviews in Endocrine and Metabolic Disorders. 18 (4): 517–532. doi:10.1007/s11154-017-9433-0. ISSN 1573-2606. PMID 28916903.
- Coggshall, Kathleen; Tello, Tiffany L.; North, Jeffrey P.; Yu, Siegrid S. (2018-03-01). "Merkel cell carcinoma: An update and review: Pathogenesis, diagnosis, and staging". Journal of the American Academy of Dermatology. 78 (3): 433–442. doi:10.1016/j.jaad.2017.12.001. ISSN 0190-9622. PMID 29229574.
- "Sun protection". World Health Organization. Retrieved 2018-03-28.
- "Prevention Guidelines - SkinCancer.org". www.skincancer.org. Retrieved 2018-03-28.
- "Prevent skin cancer | American Academy of Dermatology". www.aad.org. Retrieved 2018-03-28.
- Bichakjian, Christopher K.; Olencki, Thomas; Aasi, Sumaira Z.; Alam, Murad; Andersen, James S.; Blitzblau, Rachel; Bowen, Glen M.; Contreras, Carlo M.; Daniels, Gregory A.; Decker, Roy; Farma, Jeffrey M. (2018). "Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology". Journal of the National Comprehensive Cancer Network. 16 (6): 742–774. doi:10.6004/jnccn.2018.0055. ISSN 1540-1405. PMID 29891526.
- Chen MM, Roman SA, Sosa JA, Judson BL (February 2015). "The role of adjuvant therapy in the management of head and neck merkel cell carcinoma: an analysis of 4815 patients". JAMA Otolaryngology–Head & Neck Surgery. 141 (2): 137–41. doi:10.1001/jamaoto.2014.3052. PMID 25474617.
- Gupta SG, Wang LC, Peñas PF, Gellenthin M, Lee SJ, Nghiem P (June 2006). "Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature". Archives of Dermatology. 142 (6): 685–90. doi:10.1001/archderm.142.6.685. PMID 16785370.
- Becker, Jürgen C.; Stang, Andreas; DeCaprio, James A.; Cerroni, Lorenzo; Lebbé, Celeste; Veness, Michael; Nghiem, Paul (2017-10-26). "Merkel cell carcinoma". Nature Reviews. Disease Primers. 3: 17077. doi:10.1038/nrdp.2017.77. ISSN 2056-676X. PMC 6054450. PMID 29072302.
- Harms, Paul W.; Harms, Kelly L.; Moore, Patrick S.; DeCaprio, James A.; Nghiem, Paul; Wong, Michael K. K.; Brownell, Isaac; International Workshop on Merkel Cell Carcinoma Research (IWMCC) Working Group (December 2018). "The biology and treatment of Merkel cell carcinoma: current understanding and research priorities". Nature Reviews. Clinical Oncology. 15 (12): 763–776. doi:10.1038/s41571-018-0103-2. ISSN 1759-4782. PMC 6319370. PMID 30287935.
- Paulson, Kelly G.; Iyer, Jayasri G.; Blom, Astrid; Warton, E. Margaret; Sokil, Monica; Yelistratova, Lola; Schuman, Louise; Nagase, Kotaro; Bhatia, Shailender; Asgari, Maryam M.; Nghiem, Paul (March 2013). "Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage". The Journal of Investigative Dermatology. 133 (3): 642–646. doi:10.1038/jid.2012.388. ISSN 1523-1747. PMC 3570636. PMID 23190897.
- Topalian, Suzanne L.; Drake, Charles G.; Pardoll, Drew M. (2015-04-13). "Immune checkpoint blockade: a common denominator approach to cancer therapy". Cancer Cell. 27 (4): 450–461. doi:10.1016/j.ccell.2015.03.001. ISSN 1878-3686. PMC 4400238. PMID 25858804.
- FDA approves first treatment for rare form of skin cancer FDA News Release, March 23, 2017
- FDA News Release, December 19, 2018
- Munde PB, Khandekar SP, Dive AM, Sharma A (September 2013). "Pathophysiology of merkel cell". Journal of Oral and Maxillofacial Pathology. 17 (3): 408–12. doi:10.4103/0973-029x.125208. PMC 3927344. PMID 24574661.
- AJCC cancer staging manual. Amin, Mahul B.,, Edge, Stephen B.,, American Joint Committee on Cancer (Eighth ed.). Switzerland. 2018-03-30. ISBN 978-3-319-40617-6. OCLC 961218414.CS1 maint: others (link)
- "PDQ (Physician Data Query)". JNCI Journal of the National Cancer Institute. 105 (21): 1592–1593. 2013-10-18. doi:10.1093/jnci/djt327. ISSN 0027-8874.
- Emge, Drew A.; Cardones, Adela R. (October 2019). "Updates on Merkel Cell Carcinoma". Dermatologic Clinics. 37 (4): 489–503. doi:10.1016/j.det.2019.06.002. PMID 31466589.
- "Survival Rates for Merkel Cell Carcinoma, by Stage". www.cancer.org. Retrieved 2018-03-03.
- Sihto H, Kukko H, Koljonen V, Sankila R, Böhling T, Joensuu H (July 2011). "Merkel cell polyomavirus infection, large T antigen, retinoblastoma protein and outcome in Merkel cell carcinoma". Clinical Cancer Research. 17 (14): 4806–13. doi:10.1158/1078-0432.CCR-10-3363. PMID 21642382.
- Stetsenko GY, Malekirad J, Paulson KG, Iyer JG, Thibodeau RM, Nagase K, et al. (December 2013). "p63 expression in Merkel cell carcinoma predicts poorer survival yet may have limited clinical utility". American Journal of Clinical Pathology. 140 (6): 838–44. doi:10.1309/AJCPE4PK6CTBNQJY. PMC 4074520. PMID 24225752.
- Paulson KG, Iyer JG, Tegeder AR, Thibodeau R, Schelter J, Koba S, et al. (April 2011). "Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival". Journal of Clinical Oncology. 29 (12): 1539–46. doi:10.1200/JCO.2010.30.6308. PMC 3082974. PMID 21422430.
- Andea AA, Coit DG, Amin B, Busam KJ (November 2008). "Merkel cell carcinoma: histologic features and prognosis". Cancer. 113 (9): 2549–58. doi:10.1002/cncr.23874. PMID 18798233.
- Asgari MM, Sokil MM, Warton EM, Iyer J, Paulson KG, Nghiem P (July 2014). "Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma". JAMA Dermatology. 150 (7): 716–23. doi:10.1001/jamadermatol.2013.8116. PMC 4141075. PMID 24807619.
- Paulson KG, Bhatia S (June 2018). "Advances in Immunotherapy for Metastatic Merkel Cell Carcinoma: A Clinician's Guide". Journal of the National Comprehensive Cancer Network. 16 (6): 782–790. doi:10.6004/jnccn.2018.7049. PMID 29891528.
- Paulson KG, Lewis CW, Redman MW, Simonson WT, Lisberg A, Ritter D, et al. (April 2017). "Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: A prospective validation study". Cancer. 123 (8): 1464–1474. doi:10.1002/cncr.30475. PMC 5384867. PMID 27925665.
- Paulson KG, Park SY, Vandeven NA, Lachance K, Thomas H, Chapuis AG, et al. (March 2018). "Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics". Journal of the American Academy of Dermatology. 78 (3): 457–463.e2. doi:10.1016/j.jaad.2017.10.028. PMC 5815902. PMID 29102486.
- Hodgson NC (January 2005). "Merkel cell carcinoma: changing incidence trends". Journal of Surgical Oncology. 89 (1): 1–4. doi:10.1002/jso.20167. PMID 15611998.
- Howard RA, Dores GM, Curtis RE, Anderson WF, Travis LB (August 2006). "Merkel cell carcinoma and multiple primary cancers". Cancer Epidemiology, Biomarkers & Prevention. 15 (8): 1545–9. doi:10.1158/1055-9965.EPI-05-0895. PMID 16896047.
- Hasan S, Liu L, Triplet J, Li Z, Mansur D (November 2013). "The role of postoperative radiation and chemoradiation in merkel cell carcinoma: a systematic review of the literature". Frontiers in Oncology. 3: 276. doi:10.3389/fonc.2013.00276. PMC 3827544. PMID 24294591.
- Clarke CA, Robbins HA, Tatalovich Z, Lynch CF, Pawlish KS, Finch JL, et al. (February 2015). "Risk of merkel cell carcinoma after solid organ transplantation". Journal of the National Cancer Institute. 107 (2): dju382. doi:10.1093/jnci/dju382. PMC 4311175. PMID 25575645.
- Keepnews P (12 September 2007). "Joe Zawinul, 75, Jazz Fusion Pioneer, Dies". The New York Times.
- Obituaries, The Daily Telegraph, London, UK, 11 June 2018, pg27
External links

- National Cancer Institute. "Merkel Cell Carcinoma". National Institutes of Health (US). Archived from the original on 2010-12-21. Retrieved 2011-01-20.
| Classification | |
|---|---|
| External resources |