Mequinol
Mequinol, MeHQ or 4-methoxyphenol, is a phenol used in dermatology[1] and organic chemistry.[2]
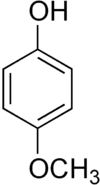 | |
| Clinical data | |
|---|---|
| Other names | 4-Hydroxyanisole; para-Guaiacol |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a682437 |
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.246 |
| Chemical and physical data | |
| Formula | C7H8O2 |
| Molar mass | 124.13722 g/mol g·mol−1 |
| Density | 1.55 g/cm3 |
| Melting point | 52.5 °C (126.5 °F) |
| Boiling point | 243 °C (469 °F) |
InChI
| |
| (verify) | |
Uses
Dermatology
Mequinol is a common active ingredient in topical drugs used for skin depigmentation. As a topical drug mequinol is often mixed with tretinoin, a topical retinoid. A common formulation for this drug is an ethanolic solution of 2% mequinol and 0.01% tretinoin by mass.[1] Dermatologists commonly prescribe the drug to treat solar lentigines, liver spots, or age spots.
Lower dosages of mequinol have been used in conjunction with a Q-switched laser to depigment skin in patients with disseminated idiopathic vitiligo.[3]
Organic chemistry
In organic chemistry 4-methoxyphenol is used as a polymerisation inhibitor (e.g. acrylates or styrene monomers).[2]
Preparation
4-Methoxyphenol is produced from p-benzoquinone and methanol via a free radical reaction.[4][5]
Safety
People can be exposed to 4-methoxyphenol in the workplace by breathing it in, skin absorption, swallowing it, skin contact, and eye contact. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 5 mg/m3 over an 8-hour workday.[6]
See also
- Monobenzone (benzyloxyphenol)
- Hydroquinone
- Guaiacol
- 2-Hydroxy-5-methoxybenzaldehyde
References
- Stiefel Laboratories, Inc. "Full Prescribing Information" (PDF). US Food and Drug Admistration. Retrieved 2 January 2015.
- Hudnall, Phillip M. "Hydroquinone". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_499.
- Komen, L.; Zwertbroek, L.; Burger, S.J.; van der Veen, J.P.W.; de Rie, M.A.; Wolkerstorfer, A. (2013). "Q-switched laser depigmentation in vitiligo, most effective in active disease". British Journal of Dermatology. 169 (6): 1246–1251. doi:10.1111/bjd.12571. PMID 23909405.
- Cristian, Gambarotti; Lucio, Melone; Carlo, Punta; Suresh Udhavrao, Shisodia (2013). "Selective Monoetherification of 1,4-Hydroquinone Promoted by NaNO2". Current Organic Chemistry. 17 (10): 1108–1113. doi:10.2174/1385272811317100011.
- US 4933504A, Correale, Mariano; Pietro Panseri & Ugo Romano et al., "Process for the preparation of mono-ethers of hydroquinones"
- "4-Methoxyphenol". NIOSH Pocket Guide to Chemical Hazards. CDC. Retrieved 2015-11-20.