Advanced maternal age
Advanced maternal age, in a broad sense, is the instance of a woman being of an older age at a stage of reproduction, although there are various definitions of specific age and stage of reproduction.[1] The variability in definitions is in part explained by the effects of increasing age occurring as a continuum rather than as a threshold effect.[1]
In Western, Northern, and Southern Europe, first-time mothers are on average 27 to 29 years old, up from 23 to 25 years at the start of the 1970s. In a number of European countries (Spain), the mean age of women at first childbirth has crossed the 30 year threshold.[2]
This process is not restricted to Europe. Asia, Japan and the United States are all seeing average age at first birth on the rise, and increasingly the process is spreading to countries in the developing world like China, Turkey and Iran. In the U.S., the average age of first childbirth was 26.6 in 2016.[3]
Advanced maternal age is associated with adverse reproductive effects such as increased risk of infertility,[4] and that the children have chromosomal abnormalities.[5] The corresponding paternal age effect is less pronounced.[6][7]
In present generations it is more common to have children at an older age. Several factors may influence the decisions of parents when having their first baby. Such factors include educational, social and economic status.
History
Having children later was not exceptional in the past, when families were larger and women often continued bearing children until the end of their reproductive age. What is so radical about this recent transformation is that it is the age at which women give birth to their first child which is becoming comparatively high, leaving an ever more constricted window of biological opportunity for second and subsequent children, should they be desired. Unsurprisingly, high first-birth ages and high rates of birth postponement are associated with the arrival of low, and lowest-low fertility.
This association has now become especially clear, since the postponement of first births in a number of countries has now continued unabated for more than three decades, and has become one of the most prominent characteristics of fertility patterns in developed societies. A variety of authors (in particular Lesthaeghe) have argued that fertility postponement constitutes the ‘hallmark’ of what has become known as the “second demographic transition”.
Others have proposed that the postponement process itself constitutes a separate 'third transition'.[8] On this latter view, modern developed societies exhibit a kind of dual fertility pattern, with the majority of births being concentrated either among very young or increasingly older mothers. This is sometimes known as the 'rectangularisation' of fertility patterns.
Examples
In the USA, the average age at which women bore their first child advanced from 21.4 years old in 1970[9] to 26.6 in 2016.[3]
The German Federal Institute for Population Research claimed in 2015 the percentage for women with an age of at least 35 giving birth to a child was 25.9%. This figure rose from 7.6% in 1981.[10]
Possible factors that influence childbearing age
There are many factors that may influence childbearing age in women, although they are mostly correlations without certain causations. For instance, older maternal age at first childbirth is associated with higher educational attainment and income.[11]
Two studies show that generous parental leave allowances in Britain encourage young motherhood and that parental-leave allowance reduces postponement in Sweden.[12]
Effects
Decreased fertility
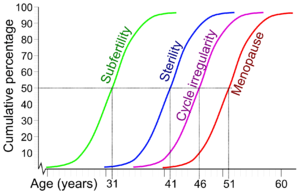
A woman's fertility peaks lasts during the twenties and first half of thirties, after which it starts to decline, with advanced maternal age causing an increased risk of female infertility.
According to Henri Leridon, PhD, an epidemiologist with the French Institute of Health and Medical Research, of women trying to get pregnant, without using fertility drugs or in vitro fertilization:[4]
- At age 30
- 75% will have a conception ending in a live birth within one year
- 91% will have a conception ending in a live birth within four years.
- At age 35
- 66% will have a conception ending in a live birth within one year
- 84% will have a conception ending in a live birth within four years.
- At age 40
- 44% will have a conception ending in a live birth within one year
- 64% will have a conception ending in a live birth within four years.[4]
Risk of birth defects
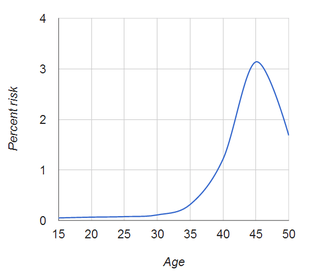
A woman's risk of having a baby with chromosomal abnormalities increases with her age. Down syndrome is the most common chromosomal birth defect, and a woman's risk of having a baby with Down syndrome is:[5]
- At age 20, 1 in 1,441
- At age 25, 1 in 1,383
- At age 30, 1 in 959
- At age 35, 1 in 338
- At age 40, 1 in 84
- At age 45, 1 in 32
- At age 50, 1 in 44
Other effects
Advanced maternal age is associated with adverse outcomes in the perinatal period, which may be caused by detrimental effects on decidual and placental development.[14]
The risk of the mother dying before the child becomes an adult increases by more advanced maternal age, such as can be demonstrated by the following data from France in 2007:[15]
| Maternal age at childbirth | 20 | 25 | 30 | 35 | 40 | 45 |
|---|---|---|---|---|---|---|
| Risk of mother not surviving until child's 18th birthday (in %)[15] | 0.6 | 1.0 | 1.6 | 2.6 | 3.8 | 5.5 |
The above table is not to be confused with maternal mortality.
Advanced maternal age continues to be associated with a range of adverse pregnancy outcomes including low birth weight, pre-term birth, stillbirth, unexplained fetal death, and increased rates of Caesarean section. However, over time, improvements in (and improvements in access to) medical services and social resources have decreased the negative association between older maternal age and low birth weight.[16]
On the other hand, advanced maternal age is associated with a more stable family environment, higher socio-economic position, higher income and better living conditions, as well as better parenting practices,[15] (including better disciplinary methods[17]). A qualitative study on couples in the United States who used in-vitro fertilization to conceive their first child when the woman was aged 40 or older at the time of delivery found that 72% of the women and 57% of the men believed that they had enhanced emotional preparedness for parenting which benefitted both their children and themselves.[18] In quantitative studies, mother’s older age at first birth has been associated with increases in children’s psychiatric health,[19] language skills,[19] cognitive ability,[20] and fewer social and emotional difficulties.[17] Further, a study in the United Kingdom showed that older maternal age at first birth was associated with fewer hospital admissions and fewer unintentional injuries for children up to age 5 and a greater likelihood of having had all of their immunizations by 9 months of age– all outcomes used as indicators of child wellbeing in reports from the World Health Organisation.[21] Finally, although older maternal age doesn’t necessarily imply older paternal age, researchers have suggested links between older paternal age and improved child outcomes, including increased IQ and educational attainment[22] and increased telomeric length, which is associated with greater longevity.[23] See also: Paternal age effect. However, it is more or less uncertain whether these entities are effects of advanced maternal age, are contributors to advanced maternal age, or common effects of a certain state such as personality type.
Changes in interpregnancy interval
Kalberer et al.[24] have shown that despite the older maternal age at birth of the first child, the time span between the birth of the first and the second child (= interpregnancy interval) decreased over the last decades. If purely biological factors were at work, it could be argued that interpregnancy interval should have increased, as fertility declines with age, which would make it harder for the woman to get a second child after postponed birth of the first one. This not being the case shows that sociologic factors (see above) prime over biological factors in determining interpregnancy interval.
With technology developments cases of post-menopausal pregnancies have occurred, and there are several known cases of older women carrying a pregnancy to term, usually with in vitro fertilization of a donor egg. A 61-year-old Brazilian woman with implantation of a donor egg expected gave birth to twins in October 2012.[25][26]
Ovarian aging
As women age, they experience a decline in reproductive performance leading to menopause. This decline is tied to a decline in the number of ovarian follicles. Although about 1 million oocytes are present at birth in the human ovary, only about 500 (about 0.05%) of these ovulate, and the rest do not (ovarian follicle atresia). The decline in ovarian reserve appears to occur at a constantly increasing rate with age,[27] and leads to nearly complete exhaustion of the reserve by about age 51. As ovarian reserve and fertility decline with age, there is also a parallel increase in pregnancy failure and meiotic errors resulting in chromosomally abnormal conceptions.
Titus et al.[28] have proposed an explanation for the decline in ovarian reserve with age. They showed that as women age, double-strand breaks accumulate in the DNA of their primordial follicles. Primordial follicles are immature primary oocytes surrounded by a single layer of granulosa cells. An enzyme system is present in oocytes that normally accurately repairs DNA double-strand breaks. This repair system is referred to as homologous recombinational repair, and it is especially active during meiosis. Meiosis is the general process by which germ cells are formed in eukaryotes, and it appears to be an adaptation for efficiently removing damages in germ line DNA by homologous recombinational repair (see Origin and function of meiosis). Human primary oocytes are present at an intermediate stage of meiosis, that is prophase I (see Oogenesis). Titus et al.[28] also showed that expression of four key DNA repair genes that are necessary for homologous recombinational repair (BRCA1, MRE11, Rad51 and ATM) decline in oocytes with age. This age-related decline in ability to repair double-strand damages can account for the accumulation of these damages, which then likely contributes to the decline in ovarian reserve.
Women with an inherited mutation in the DNA repair gene BRCA1 undergo menopause prematurely,[29] suggesting that naturally occurring DNA damages in oocytes are repaired less efficiently in these women, and this inefficiency leads to early reproductive failure. Genomic data from about 70,000 women were analyzed to identify protein-coding variation associated with age at natural menopause.[30] Pathway analyses identified a major association with DNA damage response genes, particularly those expressed during meiosis and including a common coding variant in the BRCA1 gene.
See also
- List of countries by age at first marriage
- Childlessness
- Fertility factor (demography)
- Pregnancy over age 50
- Teenage pregnancy
References
Citations
- Effect of advanced age on fertility and pregnancy in women at UpToDate. Author: Ruth C Fretts. Section Editor: Louise Wilkins-Haug. Deputy Editor: Vanessa A Barss. This topic last updated: Dec 3, 2012.
- "SF2.3: Mean age of mothers at first childbirth" (PDF). Archived from the original (PDF) on 2014-12-22. Retrieved 2014-05-27.
- https://www.cdc.gov/nchs/fastats/births.htm
- Leridon, H. (2004). "Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment". Human Reproduction. 19 (7): 1548–1553. doi:10.1093/humrep/deh304. PMID 15205397.
- Morris, JK; Mutton, DE; Alberman, E (2002). "Revised estimates of the maternal age specific live birth prevalence of Down's syndrome". Journal of Medical Screening. 9 (1): 2–6. doi:10.1136/jms.9.1.2. PMID 11943789.
- H. Tournaye, "Male Reproductive Ageing," in Bewley, Ledger, and Nikolaou, eds., Reproductive Ageing, Cambridge University Press (2009), ISBN 9781906985134 (accessed 15 November 2013)
- Kidd SA, Eskenazi B, Wyrobek AJ (2001). "Effects of male age on semen quality and fertility: a review of the literature". Fertil Steril. 75 (2): 237–48. doi:10.1016/S0015-0282(00)01679-4. PMID 11172821.
- Kohler, H. P.; Billari, F. C.; Ortega, J. A. (2002). "The emergence of lowest-low fertility in Europe during the 1990s". Population and Development Review. 28 (4): 641–680. doi:10.1111/j.1728-4457.2002.00641.x.
- Mathews, TJ. "Delayed Childbearing: More Women Are Having Their First Child Later in Life" (PDF). 2009. CDC. Retrieved 26 August 2013.
- "Archived copy" (PDF). Archived from the original (PDF) on 2017-12-22. Retrieved 2017-12-20.CS1 maint: archived copy as title (link)
- Shadyab, Aladdin H.; Gass, Margery L. S.; Stefanick, Marcia L.; Waring, Molly E.; Macera, Caroline A.; Gallo, Linda C.; Shaffer, Richard A.; Jain, Sonia; LaCroix, Andrea Z. (January 2017). "Maternal Age at Childbirth and Parity as Predictors of Longevity Among Women in the United States: The Women's Health Initiative". American Journal of Public Health. 107 (1): 113–119. doi:10.2105/AJPH.2016.303503. ISSN 0090-0036. PMC 5308150. PMID 27854529.
- Balbo, Nicoletta; Billari, Francesco C.; Mills, Melinda (2013). "Fertility in Advanced Societies: A Review of Research". European Journal of Population. 29 (1): 1–38. doi:10.1007/s10680-012-9277-y. PMC 3576563. PMID 23440941.
- te Velde, E. R. (2002). "The variability of female reproductive ageing". Human Reproduction Update. 8 (2): 141–154. doi:10.1093/humupd/8.2.141. ISSN 1355-4786. PMID 12099629.
- Nelson, S. M.; Telfer, E. E.; Anderson, R. A. (2012). "The ageing ovary and uterus: New biological insights". Human Reproduction Update. 19 (1): 67–83. doi:10.1093/humupd/dms043. PMC 3508627. PMID 23103636.
- Schmidt, L.; Sobotka, T.; Bentzen, J. G.; Nyboe Andersen, A.; on behalf of the ESHRE Reproduction Society Task Force (2011). "Demographic and medical consequences of the postponement of parenthood". Human Reproduction Update. 18 (1): 29–43. doi:10.1093/humupd/dmr040. PMID 21989171.
- Goisis, Alice; Schneider, Daniel C.; Myrskylä, Mikko (2018-09-02). "Secular changes in the association between advanced maternal age and the risk of low birth weight: A cross-cohort comparison in the UK" (PDF). Population Studies. 72 (3): 381–397. doi:10.1080/00324728.2018.1442584. ISSN 0032-4728. PMID 29582702.
- Trillingsgaard, Tea; Sommer, Dion (2018-03-04). "Associations between older maternal age, use of sanctions, and children's socio-emotional development through 7, 11, and 15 years" (PDF). European Journal of Developmental Psychology. 15 (2): 141–155. doi:10.1080/17405629.2016.1266248. ISSN 1740-5629.
- Mac Dougall, K.; Beyene, Y.; Nachtigall, R. D. (2012-04-01). "'Inconvenient biology:' advantages and disadvantages of first-time parenting after age 40 using in vitro fertilization". Human Reproduction. 27 (4): 1058–1065. doi:10.1093/humrep/des007. ISSN 0268-1161. PMC 3303492. PMID 22333985.
- Goisis, A. (2015-09-02). "How Are Children of Older Mothers Doing? Evidence from the United Kingdom" (PDF). Biodemography and Social Biology. 61 (3): 231–251. doi:10.1080/19485565.2014.1001887. ISSN 1948-5565. PMID 26652679.
- Goisis, Alice; Schneider, Daniel C; Myrskylä, Mikko (2017-06-01). "The reversing association between advanced maternal age and child cognitive ability: evidence from three UK birth cohorts". International Journal of Epidemiology. 46 (3): 850–859. doi:10.1093/ije/dyw354. ISSN 0300-5771. PMC 5837600. PMID 28177512.
- Sutcliffe, A. G.; Barnes, J.; Belsky, J.; Gardiner, J.; Melhuish, E. (2012-08-21). "The health and development of children born to older mothers in the United Kingdom: observational study using longitudinal cohort data". BMJ. 345 (aug21 1): e5116. doi:10.1136/bmj.e5116. ISSN 1756-1833. PMC 3424227. PMID 22915663.
- Janecka, M; Rijsdijk, F; Rai, D; Modabbernia, A; Reichenberg, A (2017-06-20). "Advantageous developmental outcomes of advancing paternal age". Translational Psychiatry. 7 (6): e1156. doi:10.1038/tp.2017.125. ISSN 2158-3188. PMC 5537646. PMID 28632201.
- Eisenberg, D. T. A.; Hayes, M. G.; Kuzawa, C. W. (2012-06-26). "Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants". Proceedings of the National Academy of Sciences. 109 (26): 10251–10256. doi:10.1073/pnas.1202092109. ISSN 0027-8424. PMC 3387085. PMID 22689985.
- Kalberer, U; Baud, D; Fontanet, A; Hohlfeld, P; de Ziegler, D (Dec 2009). "Birth records from Swiss married couples analyzed over the past 35 years reveal an aging of first-time mothers by 5.1 years while the interpregnancy interval has shortened" (PDF). Fertil Steril. 92 (6): 2072–3. doi:10.1016/j.fertnstert.2009.05.078. PMID 19608170.
- "Woman, 61, pregnant". The Sydney Morning Herald. 27 September 2011.
- "Antônia Letícia Asti, 61 Year-Old Brazilian Woman, Gives Birth To Twins"
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA (2008). "A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause". Hum. Reprod. 23 (3): 699–708. doi:10.1093/humrep/dem408. PMID 18192670.
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K (2013). "Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans". Sci Transl Med. 5 (172): 172ra21. doi:10.1126/scitranslmed.3004925. PMC 5130338. PMID 23408054.
- Rzepka-Górska I, Tarnowski B, Chudecka-Głaz A, Górski B, Zielińska D, Tołoczko-Grabarek A (2006). "Premature menopause in patients with BRCA1 gene mutation". Breast Cancer Res. Treat. 100 (1): 59–63. doi:10.1007/s10549-006-9220-1. PMID 16773440.
- Day FR, Ruth KS, Thompson DJ, et al. (2015). "Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair". Nat. Genet. 47 (11): 1294–303. doi:10.1038/ng.3412. PMC 4661791. PMID 26414677.
Sources
- Lorentzon, M.; et al. (2012). "Advancing Maternal Age Is Associated With Lower Bone Mineral Density In Young Adult Male Offspring". Osteoporosis International. 23 (2): 475–482. doi:10.1007/s00198-011-1558-5. PMC 3261413. PMID 21350896.
- Nilsen, AB; et al. (2012). "Characteristics Of Women Who Are Pregnant With Their First Baby At An Advanced Age". Acta Obstetricia et Gynecologica Scandinavica. 91 (3): 353–362. doi:10.1111/j.1600-0412.2011.01335.x. PMID 22150020.
- Khashan, Ali S.; et al. (2013). "Advanced Maternal Age And Adverse Pregnancy Outcome: Evidence From A Large Contemporary Cohort". PLoS ONE. 8 (2): 1–9. doi:10.1371/journal.pone.0056583. PMC 3577849. PMID 23437176.
- Jabcosson, B.; Ladfords, L.; Milsom, I. (2004). "Advanced Maternal Age and Adverse Perinatal Outcome". Obstetrics & Gynecology. 104 (4): 727–733. doi:10.1097/01.aog.0000140682.63746.be. PMID 15458893.
Further reading
- Hofmeister, Heather; Mills, Melinda; Blossfeld, Hans-Peter (2003), Globalization, Uncertainty and Women’s Mid-Career Life Courses: A Theoretical Framework. University of Bamberg, Working Papers PDF
- Lesthaeghe, R.; Neels, K. (2002). "From the first to the second demographic transition: An interpretation of the spatial continuity of demographic innovation in France, Belgium and Switzerland". European Journal of Population. 18 (4): 325–360. doi:10.1023/A:1021125800070.
- Sobotka, Tomás (2004). "Postponement of childbearing and low fertility in Europe, Dissertation". University of Groningen. Archived from the original on 2006-06-30. Retrieved 2006-04-10.
- Gavrilov, L.A., Gavrilova, N.S. Human longevity and parental age at conception. In: J.-M.Robine, T.B.L. Kirkwood, M. Allard (eds.) Sex and Longevity: Sexuality, Gender, Reproduction, Parenthood, Berlin, Heidelberg: Springer-Verlag, 2000, 7-31.
- Gavrilov, L.A., Gavrilova, N.S. Parental age at conception and offspring longevity. Reviews in Clinical Gerontology, 1997, 7: 5-12.
External links
- The Fertility Bust, Charlemagne, The Economist, February 9, 2006
- Low and Lowest-Low Fertility in Europe: Causes, Implications and Policy Options. By H-P Kohler, F.C. Billari and J.A Ortega. University of Pennsylvania, 2005.
- Postponement of childbearing and low fertility in Europe Power Point presentation by Tomas Sobotka
- InterNational Council on Infertility Information Dissemination