Marine microbial symbiosis
Microbial symbiosis in marine animals was not discovered until 1981.[1] In the time following, symbiotic relationships between marine invertebrates and chemoautotrophic bacteria have been found in a variety of ecosystems, ranging from shallow coastal waters to deep-sea hydrothermal vents. The type of marine animal vary greatly, for example, sponges, sea squirts, corals, worms, and algae all host a variety of unique symbionts.[2] Each symbiotic relationship displays a unique ecological niche, which in turn can lead to entirely new species of host species and symbiont.[1]
It is particularly interesting that it took so long to discover the marine microbial symbiosis because nearly every surface submerged in the oceans becomes covered with biofilm,[3] including a large number of living organisms. Many marine organisms display symbiotic relationships with microbes. Epibiotic bacteria have been found to live on crustacean larvae and protect them from fungal infections.[3] Other microbes in deep-sea vents have been found to prevent the settlement of barnacles and tunicate larvae.[3]
Some symbiotic relationships
Coral reef symbiosis
The most notable display of marine symbiotic relationship would be coral. Coral reefs are home to a variety of dinoflagellate symbiont,[4] these symbionts give coral its bright coloring and are vital for the survival of the reef. The symbionts provide the coral with food in exchange for protection. If the waters warm or become too acidic, the symbionts are expelled, the coral bleaches and if conditions persist the coral will die. This in turn leads to the collapse of the entire reef ecosystem[4]
Bone eating worm symbiosis
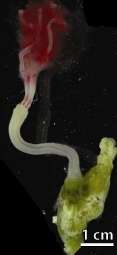
Osedax, also called the bone eating worm is a siboglinid worm from polychaete genus. It was discovered in a whalefall community on the surface of bones, in the axis of Monterey Canyon, California, in 2002. Osedax lacks a mouth, a functional gut and a trophosome. But female osedax have a vascularized root system originating from their ovisac which contains heterotrophic endosymbiotic bacterial community dominated by γ-proteobacteria clade. They use the vascularized root system to access the whale bones. The endosymbionts help the host utilize nutrients from the whale bones.[5]
Hawaiian squid and Vibrio fischeri symbiosis
Hawaiian sepiolid squid Euprymna scolopes and bacterium Vibrio fischeri also show symbiosis. In this symbiosis, symbiont not only serve the host for defense, but also shapes the host morphology. Bioluminescent V. fischeri can be found in epithelial lined crypts of the light organ of the host. Symbiosis begins as soon as a newly hatched squid finds and houses V. fischeri bacteria.
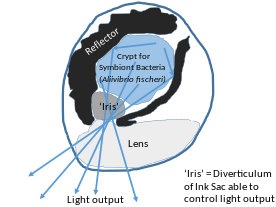
The symbiosis process begins when Peptidoglycan shed by the sea water bacteria comes in contact to the ciliated epithelial cells of the light organ. It induces mucus production in the cells. Mucus entraps bacterial cells. Antimicrobial peptides, nitric oxide and sialyted mucins in the mucus then selectively allow only V. fischeri which encode gene rscS to adhere and win over gram positive and other gram negative bacteria. The symbiotic bacteria are then guided up to the light organ via chemotaxis. After successful colonization, symbionts induce loss of mucus and ciliated sites to prevent further attachment of bacterial cells via MAMP (microbe associated molecular pattern) signalling. Also, they induce changes in protein expression in the host symbiotic tissues and modify both physiology and morphology of light organs. After bacterial cells divide and increase in population, they begin expressing enzyme luciferase as a result of quorum sensing. Luciferase enzymes produce bioluminescence.[6] Squids can then emit the luminescence from the light organ. Because Euprymna scolopes emerges only during night time, it helps them avoid predation. Bioluminescence allows them to camouflage with the light coming from moon and stars to ocean and avoid predators.[7]
Chemosynthetic symbioses in ocean
Marine environment consists of a large number of chemosynthetic symbioses in different regions of the ocean: shallow-water coastal sediments, continental slope sediments, whale and wood falls, cold seeps and deep-sea hydrothermal vents. Organisms from seven phyla (ciliophora, porifera, platyhelminthes, nematoda, mollusca, annelida and arthropoda) are known to have chemosynthetic symbiosis till now. Some of them include nematode, tube worms, clam, sponge, hydrothermal vent shrimp, worms mollusc, mussels and so on. The symbionts can be ectosymbionts or endosymbionts. Some ectosymbionts are: symbionts of polychaete worm Alvinella which occur in their dorsal surface and symbionts occurring on the mouthparts and gill chamber of the vent shrimp Rimicaris. Endosymbionts include symbionts of gastropod snails which occur in their gill tissues. In the siboglinid tube worms of the groups Monilifera, Frenulata and Vestimentifera, symbionts can be found in an interior organ called trophosome.[8]
Most of the animals in deep-sea hydrothermal vents exist in a symbiotic relationship with chemosynthetic bacteria. These chemosynthetic bacteria are found to be methane or sulphur oxidizers. [9]
Microbial biotechnology
Marine invertebrates are the hosts of a wide spectrum of bioactive metabolites, which have vast potential as drugs and research tools.[10] In many cases, microbes aid in or are responsible for marine invertebrates natural products.[10] Certain marine microbes can provide insight into the biosynthesis mechanisms of natural products, which in turn could solve the current limitations on marine drug development.[2]
References
- Cavanaugh CM (February 1994). "Microbial Symbiosis: Patterns of Diversity in the Marine Environment". American Zoologist. 34 (1): 79–89. doi:10.1093/icb/34.1.79. JSTOR 3883820.
- Li Z (April 2009). "Advances in marine microbial symbionts in the china sea and related pharmaceutical metabolites". Marine Drugs. 7 (2): 113–29. doi:10.3390/md7020113. PMC 2707038. PMID 19597576.
- Armstrong E, Yan L, Boyd KG, Wright PC, Burgess JG (October 2001). "The symbiotic role of marine microbes on living surfaces". Hydrobiologia. 461 (1–3): 37–40. doi:10.1023/A:1012756913566.
- Baker A (November 2003). "Flexibility and Specificity in Coral-Algal Symbiosis: Diversity, Ecology, and Biogeography of Symbiodinium". Annual Review of Ecology, Evolution, and Systematics. 34: 661–689. doi:10.1146/annurev.ecolsys.34.011802.132417. JSTOR 30033790.
- Goffredi SK, Orphan VJ, Rouse GW, Jahnke L, Embaye T, Turk K, Lee R, Vrijenhoek RC (September 2005). "Evolutionary innovation: a bone-eating marine symbiosis". Environmental Microbiology. 7 (9): 1369–78. doi:10.1111/j.1462-2920.2005.00824.x. PMID 16104860.
- Schwartzman JA, Ruby EG (January 2016). "A conserved chemical dialog of mutualism: lessons from squid and vibrio". Microbes and Infection. 18 (1): 1–10. doi:10.1016/j.micinf.2015.08.016. PMC 4715918. PMID 26384815.
- Nyholm SV, McFall-Ngai MJ (August 2004). "The winnowing: establishing the squid-vibrio symbiosis". Nature Reviews. Microbiology. 2 (8): 632–42. doi:10.1038/nrmicro957. PMID 15263898.
- Dubilier N, Bergin C, Lott C (October 2008). "Symbiotic diversity in marine animals: the art of harnessing chemosynthesis". Nature Reviews. Microbiology. 6 (10): 725–40. doi:10.1038/nrmicro1992. PMID 18794911.
- Petersen JM, Zielinski FU, Pape T, Seifert R, Moraru C, Amann R, Hourdez S, Girguis PR, Wankel SD, Barbe V, Pelletier E, Fink D, Borowski C, Bach W, Dubilier N (August 2011). "Hydrogen is an energy source for hydrothermal vent symbioses". Nature. 476 (7359): 176–80. Bibcode:2011Natur.476..176P. doi:10.1038/nature10325. PMID 21833083.
- Haygood MG, Schmidt EW, Davidson SK, Faulkner DJ (August 1999). "Microbial symbionts of marine invertebrates: opportunities for microbial biotechnology" (PDF). Journal of Molecular Microbiology and Biotechnology. 1 (1): 33–43. PMID 10941782.