Lipid-gated ion channels
Lipid-gated ion channels are a class of ion channels whose conductance of ions through the membrane depends directly on lipids. Classically the lipids are membrane resident anionic signaling lipids that bind to the transmembrane domain on the inner leaflet of the plasma membrane with properties of a classic ligand. However, other classes of lipid-gated channels include the mechanosensitive ion channels that respond to lipid tension, thickness, and hydrophobic mismatch.
| Lipid-gated ion-channel Kir2.2 | |
|---|---|
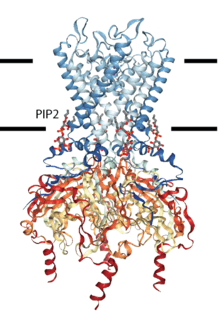 Lipid-gated ion channel | |
| Identifiers | |
| Symbol | Kir2.2 |
| OPM protein | 3SPI |
PIP2-gated channels
Phosphatidylinositol 4,5-bisphosphate (PIP2) was the first and remains the best studied lipid to gate ion channels. PIP2 is a cell membrane lipid, and its role in gating ion channels represents a novel role for the molecule,[1][2]
Kir channels: PIP2 binds to and directly activates inwardly rectifying potassium channels (Kir).[3] The lipid bound in a well defined ligand binding sitee in the transmembrane domain and cause the helices to splay opening the channel. All members of the Kir super-family of potasium channels are thought to be direclty gated by PIP.[1]
TRP channels: PIP2 regulates the conductance of most TRP channels either positively or negatively. For TRPV5, binding of PIP2 to a site in the transmembrane domain caused a conformational change that appeared to open the conduction pathway,[4] suggesting the channel is classically lipid-gated. A PIP2 compatible site was found in TRPV1 but whether the lipid alone can gate the channels has not been shown.[2] Other TRP channels that dirctly bind PIP2 are TRPM8 and TRPML.[5][6] Direct binding does not exclude PIP2 from affecting the channel by indirect mechanisms.
PA-gated channels
Phosphatidic acid (PA) recently emerged as an activator of ion channels[7]. PA directly activates TREK-1 potassium channels through a putative site in the transmembrane domain. The affinity of PA for TREK-1 is relatively weak but the enzyme PLD2 produces high local concentration of PA to activate the channel.[8][9] PA also activates the nAChR in artificial membranes. Initially, the high concentration of PA required to activate nAChR[10] suggested a related anionic lipid might activate the channel, however, the finding of local high concentration of PA activating TREK-1 may suggest otherwise.
PA binding can also influence the midpoint of voltage activation (Vmid) for voltage-activated potassium channels.[11] Depletion of PA shifted the Vmid -40 mV near resting membrane potential which could open the channel absent a change in voltage suggesting these channels may also be lipid-gated. Lipids were proposed to non-specifically gated a homologous channel from bacteria KvAP,[12] but those experiments did not rule out the anionic lipid phosphatidylglycerol from contributing specificy to gating.
Mechanosensitive channels
A specialized set of mechanosensitive ion channels is gated by lipid deformation in the membrane in response to mechanical force. A theory involving the lipid membrane, called "force from lipid", is thought to directly open ion channels.[13] These channels include the bacterial channels MscL and MscS which open in response to lytic pressure.
Channels can also respond to membrane thickness. An amphipathic helix that runs along the inner membrane of TREK-1 channels is thought to sense changes in membrane thickness and gate the channel.[14]
Localized lipid production
Local high concentrations of lipid transiently activate TREK-1 channels.[8] Theoretical estimates suggest a signaling lipid produced near an ion channel is likely in mM concentrations.[15]
References
- Hansen SB (May 2015). "Lipid agonism: The PIP2 paradigm of ligand-gated ion channels". Biochimica et Biophysica Acta. 1851 (5): 620–8. doi:10.1016/j.bbalip.2015.01.011. PMC 4540326. PMID 25633344.
- Gao Y, Cao E, Julius D, Cheng Y (June 2016). "TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action". Nature. 534 (7607): 347–51. Bibcode:2016Natur.534..347G. doi:10.1038/nature17964. PMC 4911334. PMID 27281200.
- Hansen SB, Tao X, MacKinnon R (August 2011). "Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2". Nature. 477 (7365): 495–8. Bibcode:2011Natur.477..495H. doi:10.1038/nature10370. PMC 3324908. PMID 21874019.
- Hughes TE, Pumroy RA, Yazici AT, Kasimova MA, Fluck EC, Huynh KW, Samanta A, Molugu SK, Zhou ZH, Carnevale V, Rohacs T, Moiseenkova-Bell VY (October 2018). "Structural insights on TRPV5 gating by endogenous modulators". Nature Communications. 9 (1): 4198. Bibcode:2018NatCo...9.4198H. doi:10.1038/s41467-018-06753-6. PMC 6179994. PMID 30305626.
- Fine M, Schmiege P, Li X (October 2018). "2-mediated human TRPML1 regulation". Nature Communications. 9 (1): 4192. doi:10.1038/s41467-018-06493-7. PMC 6180102. PMID 30305615.
- Yin Y, Le SC, Hsu AL, Borgnia MJ, Yang H, Lee SY (March 2019). "Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel". Science. 363 (6430): eaav9334. doi:10.1126/science.aav9334. PMC 6478609. PMID 30733385.
- Robinson, CV; Rohacs, T; Hansen, SB (3 May 2019). "Tools for Understanding Nanoscale Lipid Regulation of Ion Channels". Trends in Biochemical Sciences. doi:10.1016/j.tibs.2019.04.001. PMID 31060927.
- Comoglio Y, Levitz J, Kienzler MA, Lesage F, Isacoff EY, Sandoz G (September 2014). "Phospholipase D2 specifically regulates TREK potassium channels via direct interaction and local production of phosphatidic acid". Proceedings of the National Academy of Sciences of the United States of America. 111 (37): 13547–52. Bibcode:2014PNAS..11113547C. doi:10.1073/pnas.1407160111. PMC 4169921. PMID 25197053.
- Cabanos C, Wang M, Han X, Hansen SB (August 2017). "2 Antagonism of TREK-1 Channels". Cell Reports. 20 (6): 1287–1294. doi:10.1016/j.celrep.2017.07.034. PMC 5586213. PMID 28793254.
- Hamouda AK, Sanghvi M, Sauls D, Machu TK, Blanton MP (April 2006). "Assessing the lipid requirements of the Torpedo californica nicotinic acetylcholine receptor". Biochemistry. 45 (13): 4327–37. doi:10.1021/bi052281z. PMC 2527474. PMID 16566607.
- Hite RK, Butterwick JA, MacKinnon R (October 2014). "Phosphatidic acid modulation of Kv channel voltage sensor function". eLife. 3. doi:10.7554/eLife.04366. PMC 4212207. PMID 25285449.
- Zheng H, Liu W, Anderson LY, Jiang QX (22 March 2011). "Lipid-dependent gating of a voltage-gated potassium channel". Nature Communications. 2 (1): 250. Bibcode:2011NatCo...2E.250Z. doi:10.1038/ncomms1254. PMC 3072105. PMID 21427721.
- Teng J, Loukin S, Anishkin A, Kung C (January 2015). "The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements". Pflügers Archiv. 467 (1): 27–37. doi:10.1007/s00424-014-1530-2. PMC 4254906. PMID 24888690.
- Nayebosadri A, Petersen EN, Cabanos C, Hansen SB (2018). "A Membrane Thickness Sensor in TREK-1 Channels Transduces Mechanical Force". doi:10.2139/ssrn.3155650.
- Robinson, Carol V.; Rohacs, Tibor; Hansen, Scott B. (May 2019). "Tools for Understanding Nanoscale Lipid Regulation of Ion Channels". Trends in Biochemical Sciences. doi:10.1016/j.tibs.2019.04.001. PMID 31060927.