Linzagolix
Linzagolix (INN; developmental code names KLH-2109, OBE-2109) is a small-molecule, non-peptide, orally active gonadotropin-releasing hormone antagonist (GnRH antagonist) which is under development by Kissei Pharmaceutical and ObsEva for the treatment of uterine fibroids, endometriosis, and adenomyosis.[1][3][2] As of December 2017, it is in phase III clinical trials for uterine fibroids and phase II clinical studies for endometriosis and adenomyosis.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | KLH-2109; OBE-2109 |
| Routes of administration | By mouth[1][2] |
| Drug class | GnRH modulator; GnRH antagonist; Antigonadotropin |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
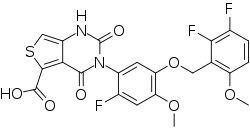
| Formula | C22H15F3N2O7S |
| Molar mass | 508.424 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
- Gonadotropin-releasing hormone receptor § Antagonists
- List of investigational hormonal agents § GnRH/gonadotropins
References
- http://adisinsight.springer.com/drugs/800032710
- Ezzati M, Carr BR (2015). "Elagolix, a novel, orally bioavailable GnRH antagonist under investigation for the treatment of endometriosis-related pain". Womens Health (Lond). 11 (1): 19–28. doi:10.2217/whe.14.68. PMID 25581052.
- Chodankar, Rohan; Allison, Jennifer (2018). "New Horizons in Fibroid Management". Current Obstetrics and Gynecology Reports. 7 (2): 106–115. doi:10.1007/s13669-018-0242-6. ISSN 2161-3303.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.