Disk diffusion test
The disk diffusion test, or agar diffusion test, or Kirby–Bauer test (disc-diffusion antibiotic susceptibility test, disc-diffusion antibiotic sensitivity test, KB test), is a test of the antibiotic sensitivity of bacteria. It uses antibiotic discs to test the extent to which bacteria are affected by those antibiotics. In this test, wafers containing antibiotics are placed on an agar plate where bacteria have been placed, and the plate is left to incubate. If an antibiotic stops the bacteria from growing or kills the bacteria, there will be an area around the wafer where the bacteria have not grown enough to be visible.[1][2] This is called a zone of inhibition.
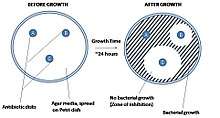
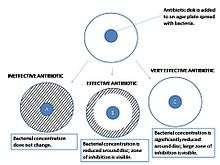
The size of this zone depends on many factors, one being how effective the antibiotic is at stopping the growth of the bacterium. Another factor that will influence the size of a zone is the diffusion of the antibiotic within the agar medium and varies based on the molecular configuration of the antibiotic. Once the zone diameter is measured it must be compared to a database of zone standards to determine if the bacterium being studied is susceptible, moderately susceptible or resistant to the antibiotic in question.
A pure bacterial culture is suspended in buffer, standardized to turbidity and swabbed uniformly across a culture plate. A filter-paper disk, impregnated with the compound to be tested, is then placed on the surface of the agar. The compound diffuses from the filter paper into the agar. The concentration of the compound will be highest next to the disk, and will decrease as distance from the disk increases. If the compound is effective against bacteria at a certain concentration, no colonies will grow where the concentration in the agar is greater than or equal to the effective concentration. This is the zone of inhibition. This along with the rate of antibiotic diffusion are used to estimate the bacteria's susceptibility to that particular antibiotic. In general, larger zones correlate with smaller minimum inhibitory concentration (MIC) of antibiotic for that bacteria. Inhibition produced by the test is compared with that produced by known concentration of a reference compound. This information can be used to choose appropriate antibiotics to combat a particular infection.[3]
Standardization
Inoculation is made with a broth culture diluted to match a 0.5 McFarland turbidity standard, which is roughly equivalent to 150 million cells per mL.

Preparation
All aspects of the Kirby–Bauer procedure are standardized to ensure consistent and accurate results. Because of this, a laboratory must adhere to these standards. The media used in Kirby–Bauer testing must be Mueller-Hinton agar at only 4 mm deep, poured into either 100 mm or 150 mm Petri dishes. The pH level of the agar must be between 7.2 and 7.4.
Incubation procedure
- Using an aseptic technique, place a sterile swab into the broth culture of a specific organism and then gently remove the excess liquid by gently pressing or rotating the swab against the inside of the tube.
- Using the swab, streak the Mueller-Hinton agar plate to form a bacterial lawn.
- To obtain uniform growth, streak the plate with the swab in one direction, rotate the plate 90° and streak the plate again in that direction.
- Repeat this rotation 3 times.
- Allow the plate to dry for approximately 5 minutes.
- Use an antibiotic disc dispenser to dispense discs containing specific antibiotics onto the plate.
- Using a flame-sterilized forceps, gently press each disc to the agar to ensure that the disc is attached to the agar.
- Plates should be incubated overnight at an incubation temperature of 37 °C (98.6 °F).[4]
Oxford penicillin cup method
Disks containing increasing antibiotic concentrations are placed on seeded bacterial lawn on the agar surface and plates are incubated. Zone sizes are measured from the edge of the disk to the end of the clear zone. Interpretation is more complicated in mixed susceptibility populations (cf. BSAC). These are plotted as linear dimensions or squares of distances as a function of the natural logarithm of antibiotic concentration in the disks. The MIC is determined from the zero intercept of a linear regression fit through the data (cf. agardiffusion.com). The intercept itself is the logarithm of the MIC. The slope of the regression line is related to the diffusion coefficient of that particular antibiotic in the agar.[6]
See also
- Etest
Further reading
- Bauer, Alfred W. (1 August 1959). "Single-Disk Antibiotic-Sensitivity Testing of Staphylococci". Archives of Internal Medicine. 104 (2): 208–16. doi:10.1001/archinte.1959.00270080034004.
- Bauer, AW; Kirby, WM; Sherris, JC; Turck, M (April 1966). "Antibiotic susceptibility testing by a standardized single disk method". American Journal of Clinical Pathology. 45 (4): 493–6. doi:10.1093/ajcp/45.4_ts.493. PMID 5325707.
- Bauer, AW; Kirby, WM; Sherris, JC; Turck, M (March 1966). "Antibiotic susceptibility testing by a standardized single disk method". Technical Bulletin of the Registry of Medical Technologists. 36 (3): 49–52. PMID 5908210.
References
- "Bacterial illumination" (PDF). Retrieved 2013-05-24.
- Brown DF, Kothari D (1975). "Comparison of antibiotic discs from different sources". J. Clin. Pathol. 28 (10): 779–83. doi:10.1136/jcp.28.10.779. PMC 475859. PMID 1214010.
- Mohanty, A. (2010): Physiochemical and antimicrobial study of polyherbal. Pharmacie globale. Vol. 4(4) 1-3.
- "Archived copy". Archived from the original on 2013-07-31. Retrieved 2013-09-01.CS1 maint: archived copy as title (link)
- Sahu B K, Antimicrobial properties of Aerial Part of Sesbania grandiflora (Linn.), The Pharmaceutical college Barpali,India,*th semester Project 2013
- Bonev, B; Hooper, J; Parisot, J (June 2008). "Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method". The Journal of Antimicrobial Chemotherapy. 61 (6): 1295–301. doi:10.1093/jac/dkn090. PMID 18339637.