Jervell and Lange-Nielsen syndrome
Jervell and Lange-Nielsen syndrome (JLNS) is a type of long QT syndrome associated with severe, bilateral sensorineural hearing loss. Long QT syndrome causes the cardiac muscle to take longer than usual to recharge between beats. If untreated, the irregular heartbeats, called arrhythmias, can lead to fainting, seizures, or sudden death. It was first described by Anton Jervell and Fred Lange-Nielsen in 1957.[2]
| Jervell and Lange-Nielsen syndrome | |
|---|---|
| Other names | Long QT interval-deafness syndrome[1] |
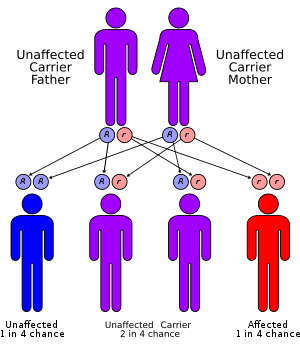 | |
| Jervell and Lange-Nielsen syndrome has an autosomal recessive pattern of inheritance. | |
| Specialty | Cardiology |
Symptoms and signs
The symptoms of this condition are:
- Congenital Deafness
- Syncope
- Seizures
- Palpitations
- Sudden cardiac death
The clinical signs are:
- Prolonged QTc on the ECG
- Bilateral sensorineural deafness
Genetics
Jervell and Lange-Nielsen syndrome is caused by mutations in the KCNE1 and KCNQ1 genes. The proteins produced by these two genes work together to form a potassium channel that transports positively charged potassium ions out of cells, which is called the slow delayed rectifier potassium current. The movement of potassium ions through these channels is critical for maintaining the normal functions of the inner ear and cardiac muscle.[3] JLNS is an autosomal recessive disorder meaning that two copies of the genetic mutation are required to produce the full syndrome. Mutations in the same genes can produce milder Romano-Ward forms of long QT syndrome if only a single copy of the genetic mutation has been inherited.
About 90% of cases of Jervell and Lange-Nielsen syndrome are caused by mutations in the KCNQ1 gene, leading to Jervell and Lange-Nielsen syndrome type 1 (JLNS1). KCNE1 mutations are responsible for the remaining 10% of cases, causing Jervell and Lange-Nielsen syndrome type 2 (JLNS2). Mutations in these genes alter the usual structure and function of potassium channels or prevent the assembly of normal channels. These changes disrupt the flow of potassium ions in the inner ear and in cardiac muscle, leading to the hearing loss and irregular heart rhythm characteristic of Jervell and Lange-Nielsen syndrome.[3]
| Type | OMIM | Gene | Notes |
| JLNS1 | 192500 | KCNQ1 | Encodes the α-subunit of the slow delayed rectifier potassium channel KV7.1 carrying the potassium current IKs. [4] |
| JLNS2 | 176261 | KCNE1 | Encodes MinK, a potassium channel β-subunit. [4] |
Diagnosis
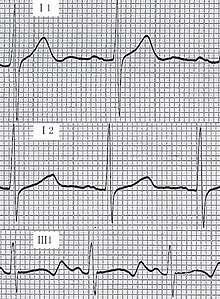
The tests used may be -
- ECG
- Holter Monitoring
- Cardiac Event Monitoring
- Hearing tests
- Genetic testing
- Electrolyte tests
- Implantable loop recorder (ILR) Monitoring
- Stress ECG/Treadmill ECG Testing
Treatment
JLNS patients with KCNQ1 mutations are particularly prone to pathological lengthening of the QT interval, which predisposes them to episodes of torsades de pointes and sudden cardiac death. In this context, if the patient has had syncopal episodes or history of cardiac arrest, an implantable cardiac defibrillator should be used in addition to a beta blocker such as propranolol.[3]
Prognosis
The risk of arrhythmias is higher for those with Jervell and Lange-Nielsen syndrome than other forms of long QT syndrome.[5] Although this risk is dependent on the underlying genetic defect and degree of QT prolongation, without treatment more than 50% of those affected will die before the age of 15.[6] However, treatment with beta blockers markedly reduces the risk of death, as does, in selected cases, implantation of a defibrillator.[6]
Epidemiology
Jervell and Lange-Nielsen syndrome affects an estimated one in 166,000 to 625,000 children, and is responsible for less than 10% of all cases of long QT syndrome. It has a markedly higher incidence in Norway and Sweden at up to one per 200,000.[3]
References
- RESERVED, INSERM US14-- ALL RIGHTS. "Orphanet: Jervell and Lange Nielsen syndrome". www.orpha.net. Retrieved 28 May 2019.
- Jervell, A.; Lange-Nielsen, F. (1957). "Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval, and sudden death". American Heart Journal. 54 (1): 59–68. doi:10.1016/0002-8703(57)90079-0. PMID 13435203.
- Tranebjaerg, L.; Samson, R.A.; Green, G.E. (1993). "Jervell and Lange-Nielsen Syndrome". GeneReviews. University of Washington, Seattle. Retrieved 29 November 2013.
- Giudicessi, John R.; Wilde, Arthur A. M.; Ackerman, Michael J. (October 2018). "The genetic architecture of long QT syndrome: A critical reappraisal". Trends in Cardiovascular Medicine. 28 (7): 453–464. doi:10.1016/j.tcm.2018.03.003. ISSN 1873-2615. PMC 6590899. PMID 29661707.
- Giudicessi, John R.; Ackerman, Michael J. (October 2013). "Genotype- and phenotype-guided management of congenital long QT syndrome". Current Problems in Cardiology. 38 (10): 417–455. doi:10.1016/j.cpcardiol.2013.08.001. ISSN 1535-6280. PMC 3940076. PMID 24093767.
- Pabba, Krishna; Chakraborty, Rebanta K. (2019), "Jervell and Lange Nielsen Syndrome", StatPearls, StatPearls Publishing, PMID 30725985, retrieved 2019-06-17
External links
| Classification | |
|---|---|
| External resources |
|
This article incorporates public domain text from The U.S. National Library of Medicine