Collecting duct system
The collecting duct system of the kidney consists of a series of tubules and ducts that physically connect nephrons to a minor calyx or directly to the renal pelvis. The collecting duct system participates in electrolyte and fluid balance through reabsorption and excretion, processes regulated by the hormones aldosterone and vasopressin (antidiuretic hormone).
| Collecting duct system | |
|---|---|
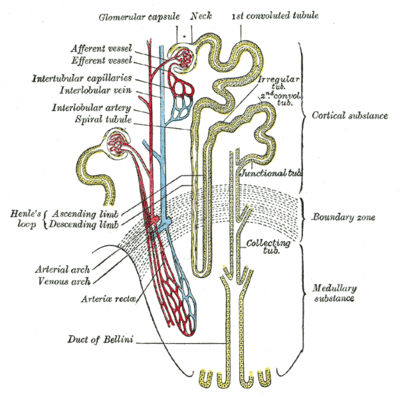 Scheme of renal tubule and its vascular supply. | |
| Details | |
| Location | Kidney |
| Identifiers | |
| Latin | tubulus renalis colligens |
| MeSH | D007685 |
| FMA | 265239 |
| Anatomical terminology | |
There are several components of the collecting duct system, including the connecting tubules, cortical collecting ducts, and medullary collecting ducts.
Structure
Segments
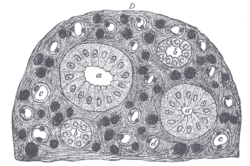
The segments of the system are as follows:
| Segment | Description |
|---|---|
| connecting tubule | Connects distal convoluted tubule to the cortical collecting duct |
| initial collecting tubule | Before convergence of nephrons |
| cortical collecting ducts | |
| medullary collecting ducts | |
| papillary ducts |
Connecting tubule
With respect to the renal corpuscle, the "connecting tubule" is the most proximal part of the collecting duct system. It is adjacent to the distal convoluted tubule, the most distal segment of the renal tubule. Connecting tubules from several adjacent nephrons merge to form cortical collecting tubules, and these may join to form cortical collecting ducts. Connecting tubules of some juxtamedullary nephrons may arch upward, forming an arcade.
The connecting tubule derives from the metanephric blastema, but the rest of the system derives from the ureteric bud. Because of this, some sources group the connecting tubule as part of the nephron, rather than grouping it with the collecting duct system.
The initial collecting tubule is a segment with a constitution similar as the collecting duct, but before the convergence with other tubules.
The "cortical collecting ducts" receive filtrate from multiple initial collecting tubules and descend into the renal medulla to form medullary collecting ducts.
Medullary collecting duct
"Medullary collecting ducts" are divided into outer and inner segments, the latter reaching more deeply into the medulla. The variable reabsorption of water and, depending on fluid balances and hormonal influences, the reabsorption or secretion of sodium, potassium, hydrogen and bicarbonate ion continues here. Urea passively transports out of duct here and creates 500mOsm gradient.
The outer segment of the medullary collecting duct follows the cortical collecting duct. It reaches the level of the renal medulla where the thin descending limb of loop of Henle borders with the thick ascending limb of loop of Henle[1]:837
The inner segment is the part of the collecting duct system between the outer segment and the papillary ducts.
Papillary duct
The terminal portions of the medullary collecting ducts are the "papillary ducts", which end at the renal papilla and empty into a minor calyx.
Cells
Each component of the collecting duct system contains two cell types, intercalated cells and a segment-specific cell type:
- For the tubules, this specific cell type is the connecting tubule cell
- For the collecting ducts, it is the principal cell. The inner medullary collecting ducts contain an additional cell type, called the inner medullary collecting duct cell.
Principal cells
The principal cell mediates the collecting duct's influence on sodium and potassium balance via sodium channels and potassium channels located on the cell's apical membrane. Aldosterone determines expression of sodium channels (especially the ENaC). Increases in aldosterone increase expression of luminal sodium channels.[2] Aldosterone also increases the number of Na⁺/K⁺-ATPase pumps [3]:949 that allow increased sodium reabsorption and potassium excretion.[3]:336 Vasopressin determines the expression of aquaporin channels that provide a physical pathway for water to pass through the principal cells.[4] Together, aldosterone and vasopressin let the principal cell control the quantity of water that is reabsorbed.
Intercalated cells
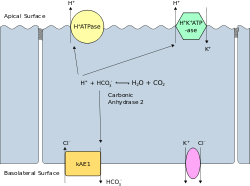
Intercalated cells come in α, β, and non-α non-β varieties and participate in acid-base homeostasis.[5][6]
| Type of cell | Secretes | Reabsorbs |
| α-intercalated cells | acid (via an apical H+-ATPase and H+/K+ exchanger) in the form of hydrogen ions | bicarbonate (via band 3, a basolateral Cl−/HCO3− exchanger)[7] |
| β-intercalated cells | bicarbonate (via pendrin a specialised apical Cl−/HCO3−) | acid (via a basal H+-ATPase) |
| non-α non-β intercalated cells | acid (via an apical H+-ATPase and H+/K+ exchanger) and bicarbonate (via pendrin)[8][9] | - |
For their contribution to acid-base homeostasis, the intercalated cells play important roles in the kidney's response to acidosis and alkalosis. Damage to the α-intercalated cell's ability to secrete acid can result in distal renal tubular acidosis (RTA type I, classical RTA)(reference). The intercalated cell population is also extensively modified in response to chronic lithium treatment, including the addition of a largely uncharacterized cell type which expressed markers for both intercalated and principal cells.[10][11]
Function
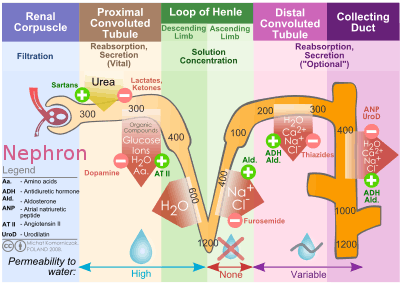
The collecting duct system is the final component of the kidney to influence the body's electrolyte and fluid balance. In humans, the system accounts for 4–5% of the kidney's reabsorption of sodium and 5% of the kidney's reabsorption of water. At times of extreme dehydration, over 24% of the filtered water may be reabsorbed in the collecting duct system.
The wide variation in water reabsorption levels for the collecting duct system reflects its dependence on hormonal activation. The collecting ducts, in particular, the outer medullary and cortical collecting ducts, are largely impermeable to water without the presence of antidiuretic hormone (ADH, or vasopressin).
- In the absence of ADH, water in the renal filtrate is left alone to enter the urine, promoting diuresis.
- When ADH is present, aquaporins allow for the reabsorption of this water, thereby inhibiting diuresis.
The collecting duct system participates in the regulation of other electrolytes, including chloride, potassium, hydrogen ions, and bicarbonate.
See also
References
- Boron, Walter F. (2005). Medical Physiology: A Cellular and Molecular Approach (updated ed.). Philadelphia: Elsevier/Saunders. ISBN 1-4160-2328-3.
- May, Anne; Puoti, Alessandro; Gaeggeler, Hans-Peter; Horisberger, Jean-Daniel; Rossier, Bernard C (1997). "Early Effect of Aldosterone on the Rate of Synthesis of the Epithelial Sodium Channel a Subunit in A6 Renal Cells" (PDF). Journal of the American Society of Nephrology. 8 (12): 1813–1822. Retrieved 21 November 2017.
- Guyton, Arthur C.; John E. Hall (2006). Textbook of Medical Physiology (11 ed.). Philadelphia: Elsevier Saunders. ISBN 0-7216-0240-1.
- Schlatter, Eberhard; Schafer, James A. (1987). "Electrophysiological studies in principal cells of rat cortical collecting tubules ADH increases the apical membrane Na+-conductance". Pflügers Archiv: European Journal of Physiology. 409: 81. doi:10.1007/BF00584753.
- Alper, S. L.; Natale, J.; Gluck, S.; Lodish, H. F.; Brown, D. (1989-07-01). "Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase". Proceedings of the National Academy of Sciences. 86 (14): 5429–5433. doi:10.1073/pnas.86.14.5429. ISSN 0027-8424. PMC 297636. PMID 2526338.
- Kim, J.; Kim, Y. H.; Cha, J. H.; Tisher, C. C.; Madsen, K. M. (January 1999). "Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse". Journal of the American Society of Nephrology: JASN. 10 (1): 1–12. ISSN 1046-6673. PMID 9890303.
- Nosek, Thomas M. Essentials of Human Physiology. Section 7/7ch07/7ch07p17 – "Intercalated Cells"
- Kim, Young-Hee; Kwon, Tae-Hwan; Frische, Sebastian; Kim, Jin; Tisher, C. Craig; Madsen, Kirsten M.; Nielsen, Søren (2002-10-01). "Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney". American Journal of Physiology. Renal Physiology. 283 (4): F744–F754. doi:10.1152/ajprenal.00037.2002. ISSN 1931-857X.
- Wall, Susan M.; Hassell, Kathryn A.; Royaux, Ines E.; Green, Eric D.; Chang, Judy Y.; Shipley, Gregory L.; Verlander, Jill W. (2003-01-01). "Localization of pendrin in mouse kidney". American Journal of Physiology. Renal Physiology. 284 (1): F229–F241. doi:10.1152/ajprenal.00147.2002. ISSN 1931-857X.
- Christensen, Birgitte Mønster; Marples, David; Kim, Young-Hee; Wang, Weidong; Frøkiær, Jørgen; Nielsen, Søren (2004-04-01). "Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI". American Journal of Physiology. Cell Physiology. 286 (4): C952–C964. doi:10.1152/ajpcell.00266.2003. ISSN 0363-6143.
- Himmel, Nathaniel J.; Wang, Yirong; Rodriguez, Daniel A.; Sun, Michael A.; Blount, Mitsi A. (2018-04-18). "Chronic lithium treatment induces novel patterns of pendrin localization and expression". American Journal of Physiology. Renal Physiology. 315 (2): F313–F322. doi:10.1152/ajprenal.00065.2018. ISSN 1931-857X. PMC 6139525. PMID 29667915.
External links
- Histology at KUMC epithel-epith04 "Collecting Duct (Kidney)"
- Histology image: 15803loa – Histology Learning System at Boston University – "Urinary System: kidney, medulla, collecting duct and ascending tubule"
- Histology image: 16013loa – Histology Learning System at Boston University – "Urinary System: kidney, H&E, collecting duct and ascending tubule"