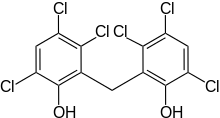
Hexachlorophene
Hexachlorophene, also known as Nabac, is an organochlorine compound that was once widely used as a disinfectant. The compound occurs as a white odorless solid, although commercial samples can be off-white and possess a slightly phenolic odor. It is insoluble in water but dissolves in acetone, ethanol, diethyl ether, and chloroform. In medicine, hexachlorophene is useful as a topical anti-infective, anti-bacterial agent, often used in soaps and toothpaste. It is also used in agriculture as a soil fungicide, plant bactericide, and acaricide.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | pHisoHex, Gamophen, Septisol, Turgex, Germa-Medica, Hexachlorophane, Almederm |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.667 |
| Chemical and physical data | |
| Formula | C13H6Cl6O2 |
| Molar mass | 406.89 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.71 g/cm3 |
| Melting point | 163 to 165 °C (325 to 329 °F) |
| Boiling point | 471 °C (880 °F) |
SMILES
| |
InChI
| |
Commercialization
US, and removal from market
In 1972, the U.S. Food and Drug Administration (FDA) halted the production and distribution of products containing more than 1% of hexachlorophene.[2] After that point, most products that contain hexachlorophene were available only with a doctor's prescription.[3] The restrictions were enacted after 15 deaths in the United States and 39 deaths in France were reported following brain damage caused by hexachlorophene.[4]
Several companies manufactured over-the-counter preparations which utilised hexachlorophene in their formulations. One product, Baby Magic Bath by The Mennen Company, was recalled in 1971, and removed from retail distribution. Immediately following its withdrawal, there was an outbreak of Staphylococcus infections in hospital nurseries across the USA.[5]
Two commercial preparations using hexachlorophene, pHisoDerm and pHisoHex, were widely used as antibacterial skin cleansers in the treatment of acne, (with pHisoDerm developed for those allergic to the active ingredients in pHisoHex). During the 1960s, both were available over the counter in the US. After the ban, pHisoDerm was reformulated without hexachlorophene, and continued to be sold over-the-counter, while pHisoHex, (which contained 3% hexachlorophene - 3 times the legal limit imposed in 1972),[4] became available as a prescription body wash. In the European Community countries during the 1970s and 1980s, pHisoHex remained available over the counter. A related product, pHisoAc, was used as a skin mask to dry and peel away acne lesions whilst pHiso-Scrub, a hexachlorophene-impregnated sponge for scrubbing, has since been discontinued. Several substitute products (including triclosan) were developed, but none had the germ-killing capability of hexachlorophene. ( Sanofi-Aventis was the sole manufacturer of pHisoHex, while The Mentholatum Company owns the pHisoDerm brand today. Sanofi-Aventis discontinued production of several forms of pHisoHex in August 2009 and discontinued all production of pHisoHex in September 2013).[6]
The formula for Dial soap was modified to remove hexachlorophene after the FDA put an end to over-the-counter availability in 1972.[3]
Another company involved in the production of products with hexachlorophene was (the now-natural) cosmetic company, J.R. Watkins Company of Winona, Minnesota. They had previously produced "Watkins Cologne for Men".
Europe
In Germany, cosmetics containing hexachlorophene have been forbidden since 1985. In Austria, sale of drugs containing the substance has been forbidden since 1990.[7]
Production
Hexacholorophene is produced by alkylation of 2,4,5-trichlorophenol with formaldehyde. Related antiseptics are prepared similarly, e.g., bromochlorophene and dichlorophene.[1]
Safety
The LD50 (oral, rat) is 59 mg/kg, indicating that the compound is relatively toxic. It is not mutagenic nor teratogenic according to Ullmann's Encyclopedia,[1] but "embryotoxic and produces some teratogenic effects" according to the International Agency for Research on Cancer.[8] 2,3,7,8-Tetrachlorodibenzodioxin (TCDD) is always a contaminant in this compound's production. Several accidents releasing many kilograms of TCDD have been reported. The reaction between 2,4,5-trichlorophenol and Formaldehyde is exothermic. If the reaction occurs without adequate cooling, TCDD is produced in significant quantities as a byproduct and contaminant. The Seveso disaster and Times Beach, Missouri contamination are two known examples of the dangers of Hexachlorophene production.
Trade names
Trade names for hexachlorophene include: Acigena, Almederm, AT7, AT17, Bilevon, Exofene, Fostril, Gamophen, G-11, Germa-Medica, Hexosan, K-34, Septisol, Surofene, M3.[9][10]
References
- Fiege, H.; Voges, H.-M.; Hamamoto, T; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313. ISBN 3-527-30673-0.
- Germicide Limit Stirs Confusion, New York Times, September 24, 1972, pg. 53.
- The Milwaukee Sentinel: "US Order Curbs Hexachlorophene" (UPI), September 23, 1972. From Google News.
- Ocala Star Banner, "15 Deaths Cited In Use of Germ Killer, Hexachlorophene" (AP), March 21, 1973. From Google News.
- Dixon, RE; Kaslow, RA; Mallison, GF; Bennett, JV (1973). "Staphylococcal disease outbreaks in hospital nurseries in the United States--December 1971 through March 1972". Pediatrics. 51 (2): 413–7. PMID 4700144.
- http://www.ashp.org/menu/DrugShortages/DrugsNoLongerAvailable/Bulletin.aspx?id=1059.
- Rechtsinformationssystem des österreichischen Bundeskanzleramtes (in German)
- "Hexachlorophene". International Agency for Research on Cancer (IARC) - Summaries & Evaluations. IPCS Inchem. 20: 241. 1998 [1979].
- "Hexachlorophene". PharmGKB. Retrieved 2012-12-28.
- Dept. of Health, Education, and Welfare (1972). "Consumer news". Office of Consumer Affairs. 2 (21): 10.CS1 maint: multiple names: authors list (link)