Thyroid function tests
Thyroid function tests (TFTs) is a collective term for blood tests used to check the function of the thyroid.[1]
| Thyroid function tests | |
|---|---|
| MeSH | D013960 |
| MedlinePlus | 003444 |
TFTs may be requested if a patient is thought to suffer from hyperthyroidism (overactive thyroid) or hypothyroidism (underactive thyroid), or to monitor the effectiveness of either thyroid-suppression or hormone replacement therapy. It is also requested routinely in conditions linked to thyroid disease, such as atrial fibrillation and anxiety disorder.
A TFT panel typically includes thyroid hormones such as thyroid-stimulating hormone (TSH, thyrotropin) and thyroxine (T4), and triiodothyronine (T3) depending on local laboratory policy.
Thyroid-stimulating hormone
Thyroid-stimulating hormone (TSH, thyrotropin) is generally increased in hypothyroidism and decreased in hyperthyroidism,[2] making it the most important test for early detection of both of these conditions.[3][4] The result of this assay is suggestive of the presence and cause of thyroid disease, since a measurement of elevated TSH generally indicates hypothyroidism, while a measurement of low TSH generally indicates hyperthyroidism.[2] However, when TSH is measured by itself, it can yield misleading results, so additional thyroid function tests must be compared with the result of this test for accurate diagnosis.[4][5][6]
TSH is produced in the pituitary gland. The production of TSH is controlled by thyrotropin-releasing hormone (TRH), which is produced in the hypothalamus. TSH levels may be suppressed by excess free T3 (fT3) or free T4 (fT4) in the blood.
History
First-generation TSH assays were done by radioimmunoassay and were introduced in 1965.[3] There were variations and improvements upon TSH radioimmunoassay, but their use declined as a new immunometric assay technique became available in the middle of the 1980s.[3][4] The new techniques were more accurate, leading to the second, third, and even fourth generations of TSH assay, with each generation possessing ten times greater functional sensitivity than the last.[7] Third generation immunometric assay methods are typically automated.[3] Fourth generation TSH immunometric assay has been developed for use in research.[4]
Current status
Third generation TSH assay is the current requirement for modern standards of care. At present, TSH testing in the United States is typically carried out with automated platforms using advanced forms of immunometric assay.[3] Nonetheless, there is currently no international standard for measurement of thyroid-stimulating hormone.[4]
Interpretation
Accurate interpretation takes a variety of factors into account, such as the thyroid hormones i.e. thyroxine (T4) and triiodothyronine (T3), current medical status (such as pregnancy[3]),[4] certain medications like propylthiouracil,[4] temporal effects including circadian rhythm[8] and hysteresis,[9] and other past medical history.[10]
Thyroid hormones
Total thyroxine
Total thyroxine is rarely measured, having been largely superseded by free thyroxine tests. Total thyroxine (Total T4) is generally elevated in hyperthyroidism and decreased in hypothyroidism.[2] It is usually slightly elevated in pregnancy secondary to increased levels of thyroid binding globulin (TBG).[2]
Total T4 is measured to see the bound and unbound levels of T4. The total T4 is less useful in cases where there could be protein abnormalities. The total T4 is less accurate due to the large amount of T4 that is bound. The total T3 is measured in clinical practice since the T3 has decreased amount that is bound as compared to T4.
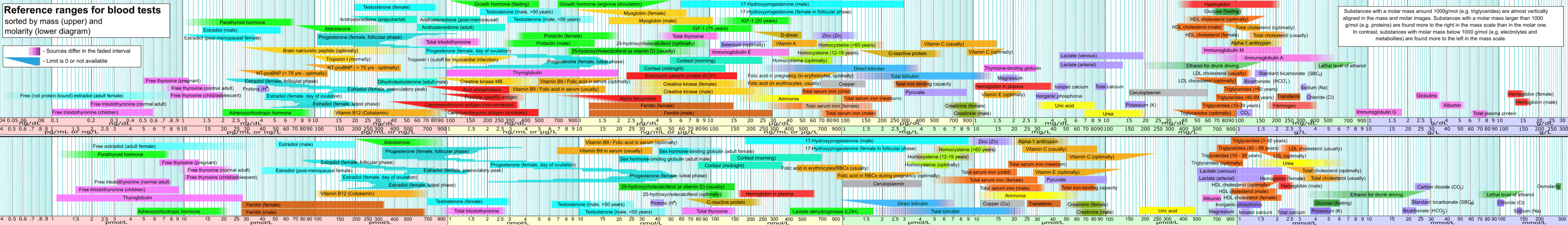
Reference ranges depend on the method of analysis. Results should always be interpreted using the range from the laboratory that performed the test. Example values are:
| Lower limit | Upper limit | Unit |
| 4,[11] 5.5[12] | 11,[11] 12.3[12] | μg/dL |
| 60[11][13] | 140,[11] 160[13] | nmol/L |
Free thyroxine
Free thyroxine (fT4) is generally elevated in hyperthyroidism and decreased in hypothyroidism.[2]
Reference ranges depend on the method of analysis. Results should always be interpreted using the range from the laboratory that performed the test. Example values are:
| Patient type | Lower limit | Upper limit | Unit |
| Normal adult | 0.7,[14] 0.8[12] | 1.4,[14] 1.5,[12] 1.8[15] | ng/dL |
| 9,[16][17] 10,[11] 12 [13] | 18,[16][17] 23[13] | pmol/L | |
| Infant 0–3 d | 2.0[14] | 5.0[14] | ng/dL |
| 26[17] | 65[17] | pmol/L | |
| Infant 3–30 d | 0.9[14] | 2.2[14] | ng/dL |
| 12[17] | 30[17] | pmol/L | |
| Child/Adolescent 31 d – 18 y | 0.8[14] | 2.0[14] | ng/dL |
| 10[17] | 26[17] | pmol/L | |
| Pregnant | 0.5[14] | 1.0[14] | ng/dL |
| 6.5[17] | 13[17] | pmol/L | |
Total triiodothyronine
Total triiodothyronine (Total T3) is rarely measured, having been largely superseded by free T3 tests. Total T3 is generally elevated in hyperthyroidism and decreased in hypothyroidism.[2]
Reference ranges depend on the method of analysis. Results should always be interpreted using the range from the laboratory that performed the test. Example values are:
| Test | Lower limit | Upper limit | Unit |
| Total triiodothyronine | 60,[12] 75[11] | 175,[11] 181[12] | ng/dL |
| 0.9,[16] 1.1[11] | 2.5,[16] 2.7[11] | nmol/L | |
Free triiodothyronine
Free triiodothyronine (fT3) is generally elevated in hyperthyroidism and decreased in hypothyroidism.[2]
Reference ranges depend on the method of analysis. Results should always be interpreted using the range from the laboratory that performed the test. Example values are:
| Patient type | Lower limit | Upper limit | Unit |
| Normal adult | 3.0[11] | 7.0[11] | pg/mL |
| 3.1[18] | 7.7[18] | pmol/L | |
| Children 2–16 y | 3.0[19] | 7.0[19] | pg/mL |
| 1.5[18] | 15.2[18] | pmol/L | |
Carrier proteins
Thyroxine-binding globulin
An increased thyroxine-binding globulin results in an increased total thyroxine and total triiodothyronine without an actual increase in hormonal activity of thyroid hormones.
Reference ranges:
| Lower limit | Upper limit | Unit |
| 12[12] | 30[12] | mg/L |
Thyroglobulin
Reference ranges:
| Lower limit | Upper limit | Unit |
| 1.5[11] | 30[11] | pmol/L |
| 1[11] | 20 [11] | μg/L |
Other binding hormones
- Transthyretin (prealbumin)
- Albumin
Protein binding function
Thyroid hormone uptake
Thyroid hormone uptake (Tuptake or T3 uptake) is a measure of the unbound thyroxine binding globulins in the blood, that is, the TBG that is unsaturated with thyroid hormone.[2] Unsaturated TBG increases with decreased levels of thyroid hormones. It is not directly related to triiodothyronine, despite the name T3 uptake.[2]
Reference ranges:
| Patient type | Lower limit | Upper limit | Unit |
| Females | 25[2] | 35[2] | % |
| In pregnancy | 15[2] | 25[2] | % |
| Males | 25[2] | 35[2] | % |
Other protein binding tests
- Thyroid Hormone Binding Ratio(THBR)
- Thyroxine-binding index (TBI)
Mixed parameters
Free thyroxine index
The Free Thyroxine Index (FTI or T7) is obtained by multiplying the total T4 with T3 uptake.[2] FTI is considered to be a more reliable indicator of thyroid status in the presence of abnormalities in plasma protein binding.[2] This test is rarely used now that reliable free thyroxine and free triiodothyronine assays are routinely available.
FTI is elevated in hyperthyroidism and decreased in hypothyroidism.[2]
| Patient type | Lower limit | Upper limit | Unit |
| Females | 1.8[2] | 5.0[2] | |
| Males | 1.3[2] | 4.2[2] | |
Structure parameters
Derived structure parameters that describe constant properties of the overall feedback control system may add useful information for special purposes, e.g. in diagnosis of nonthyroidal illness syndrome or central hypothyroidism.[20][21][22][23]
Secretory capacity (GT)
Thyroid's secretory capacity (GT, also referred to as SPINA-GT) is the maximum stimulated amount of thyroxine the thyroid can produce in one second.[24] GT is elevated in hyperthyroidism and reduced in hypothyroidism.[25]
GT is calculated with
or
: Dilution factor for T4 (reciprocal of apparent volume of distribution, 0.1 l−1)
: Clearance exponent for T4 (1.1e-6 sec−1)
K41: Dissociation constant T4-TBG (2e10 l/mol)
K42: Dissociation constant T4-TBPA (2e8 l/mol)
DT: EC50 for TSH (2.75 mU/l)[24]
| Lower limit | Upper limit | Unit |
| 1.41[24] | 8.67[24] | pmol/s |
Sum activity of peripheral deiodinases (GD)
The sum activity of peripheral deiodinases (GD, also referred to as SPINA-GD) is reduced in nonthyroidal illness with hypodeiodination.[21][22][26]
GD is obtained with
or
: Dilution factor for T3 (reciprocal of apparent volume of distribution, 0.026 l−1)
: Clearance exponent for T3 (8e-6 sec−1)
KM1: Dissociation constant of type-1-deiodinase (5e-7 mol/l)
K30: Dissociation constant T3-TBG (2e9 l/mol)[24]
| Lower limit | Upper limit | Unit |
| 20[24] | 40[24] | nmol/s |
TSH index
Jostel's TSH index (JTI or TSHI) helps to determine thyrotropic function of anterior pituitary on a quantitative level.[27] It is reduced in thyrotropic insufficiency[27] and in certain cases of non-thyroidal illness syndrome.[26]
It is calculated with
.
Additionally, a standardized form of TSH index may be calculated with
.[27]
| Parameter | Lower limit | Upper limit | Unit |
| TSHI | 1.3[27] | 4.1[27] | |
| sTSHI | -2[27] | 2[27] | |
TTSI
The Thyrotroph Thyroid Hormone Sensitivity Index (TTSI, also referred to as Thyrotroph T4 Resistance Index or TT4RI) was developed to enable fast screening for resistance to thyroid hormone.[28][29] Somewhat similar to the TSH Index it is calculated from equilibrium values for TSH and FT4, however with a different equation.
| Lower limit | Upper limit | Unit |
| 100 | 150 | |
Effects of drugs
Drugs can profoundly affect thyroid function tests. Listed below is a selection of important effects.
| Cause | Drug | Effect on hormone concentrations | Effect on structure parameters |
|---|---|---|---|
| Inhibited TSH secretion | Dopamine, L-DOPA, glucocorticoids, somatostatin | ↓T4; ↓T3; ↓TSH | ↔SPINA-GT; ↓JTI |
| Inhibited synthesis or release of thyroid hormone | Iodine, lithium | ↓T4; ↓T3; ↑TSH | ↓SPINA-GT; ↔JTI |
| Inhibited conversion of T4 to T3 (Step-up hypodeiodination) | Amiodarone, glucocorticoids, propranolol, propylthiouracil, radiographic contrast agents | ↓T3; ↑rT3; ↓, ↔, ↑T4 and fT4; ↔, ↑TSH | ↓SPINA-GD |
| Inhibited binding of T4/T3 to serum proteins | Salicylates, phenytoin, carbamazepine, furosemide, nonsteroidal anti-inflammatory agents, heparin (in vitro effect) | ↓T4; ↓T3; ↓fT4E, ↔, ↑fT4; ↔TSH | ↓T4/fT4 ratio |
| Stimulated metabolism of iodothyronines | Phenobarbital, phenytoin, carbamazepine, rifampicin | ↓T4; ↓fT4; ↔TSH | |
| Inhibited absorption of ingested T4 | Aluminium hydroxide, ferrous sulfate, cholestyramine, colestipol, iron sucralfate, soybean preparations, kayexalate | ↓T4; ↓fT4; ↑TSH | |
| Increase in concentration of T4-binding proteins | Estrogen, clofibrate, opiates (heroin, methadone), 5-fluorouracil, perphenazine | ↑T4; ↑T3; ↔fT4; ↔TSH | ↔SPINA-GT; ↔SPINA-GD; ↔JTI; ↑T4/fT4 ratio |
| Decrease in concentration of T4-binding proteins | Androgens, glucocorticoids | ↓T4; ↓T3; ↔fT4; ↔TSH | ↔SPINA-GT; ↔SPINA-GD; ↔JTI; ↓T4/fT4 ratio |
↓: reduced serum concentration or structure parameter; ↑: increased serum concentration or structure parameter; ↔: no change; TSH: Thyroid-stimulating hormone; T3: Total triiodothyronine; T4: Total thyroxine; fT4: Free thyroxine; fT3: Free triiodothyronine; rT3: Reverse triiodothyronine
See also
References
- Dayan CM (February 2001). "Interpretation of thyroid function tests". Lancet. 357 (9256): 619–24. doi:10.1016/S0140-6736(00)04060-5. PMID 11558500.
- Military Obstetrics & Gynecology > Thyroid Function Tests In turn citing: Operational Medicine 2001, Health Care in Military Settings, NAVMED P-5139, May 1, 2001, Bureau of Medicine and Surgery, Department of the Navy, 2300 E Street NW, Washington, D.C., 20372-5300 "Archived copy". Archived from the original on 25 December 2011. Retrieved 2011-12-25.CS1 maint: archived copy as title (link)
- Spencer, Carole (1 January 2013). "Assay of Thyroid Hormones and Related Substances". Thyroid Disease Manager. Retrieved 5 November 2013.
- Toft, Anthony; Beckett, Geoffrey (2005). Werner & Ingbar's The Thyroid: A Fundamental & Clinical Text (9th ed.). Philadelphia, PA: Lippincott Williams & Wilkins. pp. 329–344. ISBN 978-0-7817-5047-9.
- Hoermann, Rudolf; Midgley, John E. M.; Larisch, Rolf; Dietrich, Johannes W. (22 December 2017). "Recent Advances in Thyroid Hormone Regulation: Toward a New Paradigm for Optimal Diagnosis and Treatment". Frontiers in Endocrinology. 8: 364. doi:10.3389/fendo.2017.00364. PMC 5763098. PMID 29375474.
- Midgley, JEM; Toft, AD; Larisch, R; Dietrich, JW; Hoermann, R (18 April 2019). "Time for a reassessment of the treatment of hypothyroidism". BMC Endocrine Disorders. 19 (1): 37. doi:10.1186/s12902-019-0365-4. PMC 6471951. PMID 30999905.
- Spencer, Carole; Takeuchi, Michael; Kazarosyan, Margarita (1996). "Current status and performance goals for serum thyrotropin (TSH) assays". Clinical Chemistry. 42 (1): 141–145. Retrieved 5 November 2013.
- Hoermann, Rudolf; Midgley, John E. M.; Larisch, Rolf; Dietrich, Johannes W. (2015). "Homeostatic Control of the Thyroid–Pituitary Axis: Perspectives for Diagnosis and Treatment". Frontiers in Endocrinology. 6: 177. doi:10.3389/fendo.2015.00177. PMC 4653296. PMID 26635726.
- Leow, Melvin Khee-Shing (2016). "A Review of the Phenomenon of Hysteresis in the Hypothalamus–Pituitary–Thyroid Axis". Frontiers in Endocrinology. 7: 64. doi:10.3389/fendo.2016.00064. PMC 4905968. PMID 27379016.
- Dayan, Colin (24 February 2001). "Interpretation of thyroid function tests" (PDF). The Lancet. 357 (9256): 619–624. doi:10.1016/s0140-6736(00)04060-5. PMID 11558500. Retrieved 5 November 2013.
- Table 4: Typical reference ranges for serum assays Archived July 1, 2011, at the Wayback Machine - Thyroid Disease Manager
- Normal Reference Range Table Archived 2011-12-25 at the Wayback Machine from The University of Texas Southwestern Medical Center at Dallas. Used in Interactive Case Study Companion to Pathologic basis of disease.
- van der Watt G, Haarburger D, Berman P (July 2008). "Euthyroid patient with elevated serum free thyroxine". Clin. Chem. 54 (7): 1239–41. doi:10.1373/clinchem.2007.101428. PMID 18593963.
- Free T4; Thyroxine, Free; T4, Free Archived 2010-12-22 at the Wayback Machine UNC Health Care System
- Derived from molar values using molar mass of 776.87 g/mol
- Reference range list from Uppsala University Hospital ("Laborationslista"). Artnr 40284 Sj74a. Issued on April 22, 2008
- Derived from mass values using molar mass of 776.87 g/mol
- Derived from mass values using molar mass of 650.98 g/mol
- Cioffi M, Gazzerro P, Vietri MT, et al. (2001). "Serum concentration of free T3, free T4 and TSH in healthy children". J. Pediatr. Endocrinol. Metab. 14 (9): 1635–9. doi:10.1515/JPEM.2001.14.9.1635. PMID 11795654.
- Dietrich JW, Stachon A, Antic B, Klein HH, Hering S (2008). "The AQUA-FONTIS study: protocol of a multidisciplinary, cross-sectional and prospective longitudinal study for developing standardized diagnostics and classification of non-thyroidal illness syndrome". BMC Endocr Disord. 8: 13. doi:10.1186/1472-6823-8-13. PMC 2576461. PMID 18851740.CS1 maint: multiple names: authors list (link)
- Rosolowska-Huszcz D, Kozlowska L, Rydzewski A (August 2005). "Influence of low protein diet on nonthyroidal illness syndrome in chronic renal failure". Endocrine. 27 (3): 283–8. doi:10.1385/ENDO:27:3:283. PMID 16230785.
- Liu S, Ren J, Zhao Y, Han G, Hong Z, Yan D, Chen J, Gu G, Wang G, Wang X, Fan C, Li J (2012). "Nonthyroidal Illness Syndrome: Is it Far Away From Crohn's Disease?". J Clin Gastroenterol. 47 (2): 153–9. doi:10.1097/MCG.0b013e318254ea8a. PMID 22874844.
- Dietrich, Johannes W.; Landgrafe-Mende, Gabi; Wiora, Evelin; Chatzitomaris, Apostolos; Klein, Harald H.; Midgley, John E. M.; Hoermann, Rudolf (9 June 2016). "Calculated Parameters of Thyroid Homeostasis: Emerging Tools for Differential Diagnosis and Clinical Research". Frontiers in Endocrinology. 7: 57. doi:10.3389/fendo.2016.00057. PMC 4899439. PMID 27375554.
- Dietrich, J. W. (2002). Der Hypophysen-Schilddrüsen-Regelkreis. Berlin, Germany: Logos-Verlag Berlin. ISBN 978-3-89722-850-4. OCLC 50451543. OL 24586469M. 3897228505.
- Dietrich, J., M. Fischer, J. Jauch, E. Pantke, R. Gärtner und C. R. Pickardt (1999). "SPINA-THYR: A Novel Systems Theoretic Approach to Determine the Secretion Capacity of the Thyroid Gland." European Journal of Internal Medicine 10, Suppl. 1 (5/1999): S34.
- Fan, S; Ni, X; Wang, J; Zhang, Y; Tao, S; Chen, M; Li, Y; Li, J (February 2016). "Low Triiodothyronine Syndrome in Patients With Radiation Enteritis: Risk Factors and Clinical Outcomes an Observational Study". Medicine. 95 (6): e2640. doi:10.1097/MD.0000000000002640. PMC 4753882. PMID 26871787.
- Jostel A, Ryder WD, Shalet SM (October 2009). "The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH Index". Clin. Endocrinol. 71 (4): 529–34. doi:10.1111/j.1365-2265.2009.03534.x. PMID 19226261.
- Yagi H, Pohlenz J, Hayashi Y, Sakurai A, Refetoff S (1997). "Resistance to thyroid hormone caused by two mutant thyroid hormone receptors beta, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3'-triiodothyroinine binding affinity". J. Clin. Endocrinol. Metab. 82 (5): 1608–14. doi:10.1210/jcem.82.5.3945. PMID 9141558.
- Pohlenz J, Weiss RE, Macchia PE, Pannain S, Lau IT, Ho H, Refetoff S (1999). "Five new families with resistance to thyroid hormone not caused by mutations in the thyroid hormone receptor beta gene". J. Clin. Endocrinol. Metab. 84 (11): 3919–28. doi:10.1210/jcem.84.11.6080. PMID 10566629.
- Burtis CA, Ashwood ER, Bruns DE (2012). Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 5th edition. Elsevier Saunders. p. 1920. ISBN 978-1-4160-6164-9.
- Chatzitomaris, Apostolos; Hoermann, Rudolf; Midgley, John E.; Hering, Steffen; Urban, Aline; Dietrich, Barbara; Abood, Assjana; Klein, Harald H.; Dietrich, Johannes W. (20 July 2017). "Thyroid Allostasis–Adaptive Responses of Thyrotropic Feedback Control to Conditions of Strain, Stress, and Developmental Programming". Frontiers in Endocrinology. 8: 163. doi:10.3389/fendo.2017.00163. PMC 5517413. PMID 28775711.
Further reading
- American Thyroid Association: Thyroid Function Tests. Posted on June 4, 2012, seen on January 9, 2013.
- Thyroid function panel - Lab Tests Online
External links
- SPINA Thyr: Open source software for calculating GT and GD
- Interpretation of thyroid function tests by Dayan, Colin M. 2001. The Lancet, Vol. 357.
CDC laboratory procedure manuals
The Centers for Disease Control and Prevention has published the following laboratory procedure manuals for measuring thyroid-stimulating hormone:
- Thyroid Stimulating Hormone (TSH) (University of Washington Medical Center). September 2011. Method: Access 2 (Beckman Coulter).
- Thyroid Stimulating Hormone (TSH) (Collaborative Laboratory Services). September 2011. Method: Access 2 (Beckman Coulter).
- Thyroid Stimulating Hormone (TSH). September 2009. Method: Access 2 (Beckman Coulter).
- Lab 18 Thyroid Stimulating Hormone. 2001-2002. Method: Microparticle Enzyme Immunoassay.
- Lab 18 TSH - Thyroid Stimulating Hormone. 1999-2000. Method: Microparticle Enzyme Immunoassay.
Beckman Coulter procedure manuals
Beckman Coulter provides the equipment and reagents used in the 2009-2011 CDC manuals, and has published the following manuals for performing the procedure:
- HYPERsensitive hTSH - 3rd generation. 2010. Hosted by the University of California, San Francisco.
- Reference 33820 - HYPERsensitive hTSH 3rd generation and Fast hTSH 2nd generation
- http://www.tsh3rdgeneration.com. 2010. Hosted by manufacturer].