Fibrous dysplasia of bone
Fibrous dysplasia is a disorder where normal bone and marrow is replaced with fibrous tissue, resulting in formation of bone that is weak and prone to expansion. As a result, most complications result from fracture, deformity, functional impairment, and pain.[2] Disease occurs along a broad clinical spectrum ranging from asymptomatic, incidental lesions, to severe disabling disease. Disease can affect one bone (monostotic), multiple (polyostotic), or all bones (panostotic)[3][4] and may occur in isolation or in combination with café au lait skin macules and hyperfunctioning endocrinopathies, termed McCune–Albright syndrome.[2] More rarely, fibrous dysplasia may be associated with intramuscular myxomas, termed Mazabraud's syndrome.[5] Fibrous dysplasia is very rare, and there is no known cure. Fibrous dysplasia is not a form of cancer.
| Fibrous dysplasia | |
|---|---|
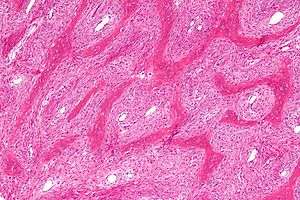 | |
| Micrograph showing fibrous dysplasia with the characteristic thin, irregular (Chinese character-like) bony trabeculae and fibrotic marrow space. H&E stain. | |
| Specialty | Medical genetics |
| Symptoms | Bone pain, bone deformities, local swelling |
| Complications | Bone fractures |
| Usual onset | Adolescence or early adulthood (monostotic), before age 10 (polyostotic) |
| Types | Monostotic (75–80% of cases),[1] polyostotic, panostotic |
| Causes | Mutations of GNAS locus |
| Frequency | 1 in 5,000 to 10,000[1] |
Presentation
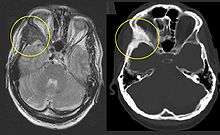
Fibrous dysplasia is a mosaic disease that can involve any part or combination of the craniofacial, axillary, and/or appendicular skeleton.[6] The type and severity of the complications therefore depend on the location and extent of the affected skeleton. The clinical spectrum is very broad, ranging from an isolated, asymptomatic monostotic lesion discovered incidentally, to severe disabling disease involving practically the entire skeleton and leading to loss of vision, hearing, and/or mobility.
Individual bone lesions typically manifest during the first few years of life and expand during childhood. The vast majority of clinically significant bone lesions are detectable by age 10 years, with few new and almost no clinically significant bone lesions appearing after age 15 years.[7] Total body scintigraphy is useful to identify and determine the extent of bone lesions, and should be performed in all patients with suspected fibrous dysplasia.[2]
Children with fibrous dysplasia in the appendicular skeleton typically present with limp, pain, and/or pathologic fractures. Frequent fractures and progressive deformity may lead to difficulties with ambulation and impaired mobility. In the craniofacial skeleton, fibrous dysplasia may present as a painless “lump” or facial asymmetry. Expansion of craniofacial lesions may lead to progressive facial deformity. In rare cases, patients may develop vision and/or hearing loss due to compromise of the optic nerves and/or auditory canals, which is more common in patients with McCune-Albright syndrome associated growth hormone excess.[8] Fibrous dysplasia commonly involves the spine, and may lead to scoliosis, which in rare instances may be severe.[9] Untreated, progressive scoliosis is one of the few features of fibrous dysplasia that can lead to early fatality.
Bone pain is a common complication of fibrous dysplasia. It may present at any age, but most commonly develops during adolescence and progresses into adulthood.[6]
Bone marrow stromal cells in fibrous dysplasia produce excess amounts of the phosphate-regulating hormone fibroblast growth factor-23 (FGF23), leading to loss of phosphate in the urine.[10] Patients with hypophosphatemia may develop rickets/osteomalacia, increased fractures, and bone pain.[11]
Pathophysiology
Fibrous dysplasia is a mosaic disease resulting from post-zygotic activating mutations of the GNAS locus at 20q13.2-q13.3, which codes for the α subunit of the Gs G-coupled protein receptor.[12] In bone, constitutive Gsα signaling results in impaired differentiation and proliferation of bone marrow stromal cells.[13] Proliferation of these cells causes replacement of normal bone and marrow with fibrous tissue. The bony trabeculae are abnormally thin and irregular, and often likened to Chinese characters (bony spicules on biopsy).
Fibrous dysplasia is not hereditary, and there has never been a case of genetic inheritance from parent to child.
Diagnosis
On x-ray, fibrous dysplasia appears as bubbly lytic lesions, or a ground glass appearance. Computerized tomography (CT) or magnetic resonance imaging (MRI) scans may be used to determine how extensively bones are affected. A bone scan uses radioactive tracers, which are injected into your bloodstream. The damaged parts of bones take up more of the tracer, which show up more brightly on the scan. A biopsy, which uses a hollow needle to remove a small piece of the affected bone for laboratory analysis, can diagnose fibrous dysplasia definitely.
Treatment
Treatment in fibrous dysplasia is mainly palliative, and is focused on managing fractures and preventing deformity. There are no medications capable of altering the disease course. Intravenous bisphosphonates may be helpful for treatment of bone pain, but there is no clear evidence that they strengthen bone lesions or prevent fractures.[14][15] Surgical techniques that are effective in other disorders, such as bone grafting, curettage, and plates and screws, are frequently ineffective in fibrous dysplasia and should be avoided.[16][17] Intramedullary rods are generally preferred for management of fractures and deformity in the lower extremities.[17] Progressive scoliosis can generally be managed with standard instrumentation and fusion techniques.[18] Surgical management in the craniofacial skeleton is complicated by frequent post-operative FD regrowth, and should focus on correction of functional deformities.[19] Prophylactic optic nerve decompression increases the risk of vision loss and is contraindicated.[20]
Managing endocrinopathies is a critical component of management in FD. All patients with fibrous dysplasia should be evaluated and treated for endocrine diseases associated with McCune–Albright syndrome. In particular untreated growth hormone excess may worsen craniofacial fibrous dysplasia and increase the risk of blindness.[21] Untreated hypophosphatemia increases bone pain and risk of fractures.[22]
See also
References
- Tafti, Dawood; Cecava, Nathan D. (2018-12-18). "Fibrous Dysplasia". NCBI Bookshelf. PMID 30422542. Retrieved 2019-12-08.
- Boyce, Alison M.; Collins, Michael T. (1993-01-01). Pagon, Roberta A.; Adam, Margaret P.; Ardinger, Holly H.; Wallace, Stephanie E.; Amemiya, Anne; Bean, Lora JH; Bird, Thomas D.; Fong, Chin-To; Mefford, Heather C. (eds.). Fibrous Dysplasia/McCune-Albright Syndrome. Seattle (WA): University of Washington, Seattle. PMID 25719192.
- Cole DE; Fraser FC; Glorieux FH; Jequier S; Marie PJ; Reade TM; Scriver CR (14 Apr 1983). "Panostotic fibrous dysplasia: a congenital disorder of bone with unusual facial appearance, bone fragility, hyperphosphatasemia, and hypophosphatemia". American Journal of Medical Genetics (4 ed.). 14 (4): 725–35. doi:10.1002/ajmg.1320140414. PMID 6846403.
- Leslie WD; Reinhold C; Rosenthall L; Tau C; Glorieux FH (July 1992). "Panostotic fibrous dysplasia. A new craniotubular dysplasia". Clinical Nuclear Medicine (7 ed.). 17 (7): 556–60. doi:10.1097/00003072-199207000-00005. PMID 1638836.
- Cabral, C. E.; Guedes, P.; Fonseca, T.; Rezende, J. F.; Cruz Júnior, L. C.; Smith, J. (1998-05-01). "Polyostotic fibrous dysplasia associated with intramuscular myxomas: Mazabraud's syndrome". Skeletal Radiology. 27 (5): 278–282. doi:10.1007/s002560050381. ISSN 0364-2348. PMID 9638839.
- Kelly, M. H.; Brillante, B.; Collins, M. T. (2008-01-01). "Pain in fibrous dysplasia of bone: age-related changes and the anatomical distribution of skeletal lesions". Osteoporosis International. 19 (1): 57–63. doi:10.1007/s00198-007-0425-x. ISSN 0937-941X. PMID 17622477.
- Hart, Elizabeth S.; Kelly, Marilyn H.; Brillante, Beth; Chen, Clara C.; Ziran, Navid; Lee, Janice S.; Feuillan, Penelope; Leet, Arabella I.; Kushner, Harvey (2007-09-01). "Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome". Journal of Bone and Mineral Research. 22 (9): 1468–1474. doi:10.1359/jbmr.070511. ISSN 0884-0431. PMID 17501668.
- Cutler, Carolee M.; Lee, Janice S.; Butman, John A.; FitzGibbon, Edmond J.; Kelly, Marilyn H.; Brillante, Beth A.; Feuillan, Penelope; Robey, Pamela G.; DuFresne, Craig R. (2006-11-01). "Long-term outcome of optic nerve encasement and optic nerve decompression in patients with fibrous dysplasia: risk factors for blindness and safety of observation". Neurosurgery. 59 (5): 1011–1017, discussion 1017–1018. doi:10.1227/01.NEU.0000254440.02736.E3. ISSN 1524-4040. PMID 17143235.
- Leet, Arabella I.; Magur, Edward; Lee, Janice S.; Wientroub, Shlomo; Robey, Pamela G.; Collins, Michael T. (2004-03-01). "Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis". The Journal of Bone and Joint Surgery. American Volume. 86-A (3): 531–537. ISSN 0021-9355. PMID 14996879.
- Riminucci, Mara; Collins, Michael T.; Fedarko, Neal S.; Cherman, Natasha; Corsi, Alessandro; White, Kenneth E.; Waguespack, Steven; Gupta, Anurag; Hannon, Tamara (2003-09-01). "FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting". The Journal of Clinical Investigation. 112 (5): 683–692. doi:10.1172/JCI18399. ISSN 0021-9738. PMC 182207. PMID 12952917.
- Leet, Arabella I.; Chebli, Caroline; Kushner, Harvey; Chen, Clara C.; Kelly, Marilyn H.; Brillante, Beth A.; Robey, Pamela G.; Bianco, Paolo; Wientroub, Shlomo (2004-04-01). "Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome". Journal of Bone and Mineral Research. 19 (4): 571–577. doi:10.1359/JBMR.0301262. ISSN 0884-0431. PMID 15005844.
- Weinstein, L. S.; Shenker, A.; Gejman, P. V.; Merino, M. J.; Friedman, E.; Spiegel, A. M. (1991-12-12). "Activating mutations of the stimulatory G protein in the McCune-Albright syndrome". The New England Journal of Medicine. 325 (24): 1688–1695. doi:10.1056/NEJM199112123252403. ISSN 0028-4793. PMID 1944469.
- Riminucci, M.; Fisher, L. W.; Shenker, A.; Spiegel, A. M.; Bianco, P.; Gehron Robey, P. (1997-12-01). "Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation". The American Journal of Pathology. 151 (6): 1587–1600. ISSN 0002-9440. PMC 1858361. PMID 9403710.
- Plotkin, Horacio; Rauch, Frank; Zeitlin, Leonid; Munns, Craig; Travers, Rose; Glorieux, Francis H. (2003-10-01). "Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone". The Journal of Clinical Endocrinology and Metabolism. 88 (10): 4569–4575. doi:10.1210/jc.2003-030050. ISSN 0021-972X. PMID 14557424.
- Boyce, Alison M.; Kelly, Marilyn H.; Brillante, Beth A.; Kushner, Harvey; Wientroub, Shlomo; Riminucci, Mara; Bianco, Paolo; Robey, Pamela G.; Collins, Michael T. (2014-11-01). "A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone". The Journal of Clinical Endocrinology and Metabolism. 99 (11): 4133–4140. doi:10.1210/jc.2014-1371. ISSN 1945-7197. PMC 4223439. PMID 25033066.
- Leet, Arabella I.; Boyce, Alison M.; Ibrahim, Khalda A.; Wientroub, Shlomo; Kushner, Harvey; Collins, Michael T. (2016-02-03). "Bone-Grafting in Polyostotic Fibrous Dysplasia". The Journal of Bone and Joint Surgery. American Volume. 98 (3): 211–219. doi:10.2106/JBJS.O.00547. ISSN 1535-1386. PMC 4732545. PMID 26842411.
- Stanton, Robert P.; Ippolito, Ernesto; Springfield, Dempsey; Lindaman, Lynn; Wientroub, Shlomo; Leet, Arabella (2012-05-24). "The surgical management of fibrous dysplasia of bone". Orphanet Journal of Rare Diseases. 7 Suppl 1: S1. doi:10.1186/1750-1172-7-S1-S1. ISSN 1750-1172. PMC 3359959. PMID 22640754.
- Leet, Arabella I.; Magur, Edward; Lee, Janice S.; Wientroub, Shlomo; Robey, Pamela G.; Collins, Michael T. (2004-03-01). "Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis". The Journal of Bone and Joint Surgery. American Volume. 86-A (3): 531–537. ISSN 0021-9355. PMID 14996879.
- Lee, J. S.; FitzGibbon, E. J.; Chen, Y. R.; Kim, H. J.; Lustig, L. R.; Akintoye, S. O.; Collins, M. T.; Kaban, L. B. (2012-05-24). "Clinical guidelines for the management of craniofacial fibrous dysplasia". Orphanet Journal of Rare Diseases. 7 Suppl 1: S2. doi:10.1186/1750-1172-7-S1-S2. ISSN 1750-1172. PMC 3359960. PMID 22640797.
- Amit, Moran; Collins, Michael T.; FitzGibbon, Edmond J.; Butman, John A.; Fliss, Dan M.; Gil, Ziv (2011-01-01). "Surgery versus watchful waiting in patients with craniofacial fibrous dysplasia--a meta-analysis". PLOS ONE. 6 (9): e25179. Bibcode:2011PLoSO...625179A. doi:10.1371/journal.pone.0025179. ISSN 1932-6203. PMC 3179490. PMID 21966448.
- Boyce, Alison M.; Glover, McKinley; Kelly, Marilyn H.; Brillante, Beth A.; Butman, John A.; Fitzgibbon, Edmond J.; Brewer, Carmen C.; Zalewski, Christopher K.; Cutler Peck, Carolee M. (2013-01-01). "Optic neuropathy in McCune-Albright syndrome: effects of early diagnosis and treatment of growth hormone excess". The Journal of Clinical Endocrinology and Metabolism. 98 (1): E126–134. doi:10.1210/jc.2012-2111. ISSN 1945-7197. PMC 3537097. PMID 23093488.
- Leet, Arabella I.; Chebli, Caroline; Kushner, Harvey; Chen, Clara C.; Kelly, Marilyn H.; Brillante, Beth A.; Robey, Pamela G.; Bianco, Paolo; Wientroub, Shlomo (2004-04-01). "Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome". Journal of Bone and Mineral Research. 19 (4): 571–577. doi:10.1359/JBMR.0301262. ISSN 0884-0431. PMID 15005844.
Further reading
- GeneReviews entry for Fibrous Dysplasia/McCune-Albright Syndrome
External links
| Classification | |
|---|---|
| External resources |