Fibroblast
A fibroblast is a type of biological cell that synthesizes the extracellular matrix and collagen,[1] produces the structural framework (stroma) for animal tissues, and plays a critical role in wound healing.[2] Fibroblasts are the most common cells of connective tissue in animals.
| Fibroblast | |
|---|---|
 NIH/3T3 Fibroblasts in cell culture | |
| Details | |
| Location | Connective tissue |
| Function | Extracellular matrix and collagen creation |
| Identifiers | |
| Latin | fibroblastus |
| MeSH | D005347 |
| TH | H2.00.03.0.01002 |
| Anatomical terms of microanatomy | |
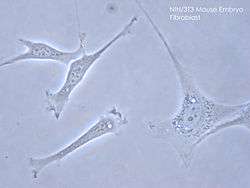
Structure
.jpg)
Fibroblasts have a branched cytoplasm surrounding an elliptical, speckled nucleus having two or more nucleoli. Active fibroblasts can be recognized by their abundant rough endoplasmic reticulum. Inactive fibroblasts (called fibrocytes) are smaller, spindle-shaped, and have a reduced amount of rough endoplasmic reticulum. Although disjointed and scattered when they have to cover a large space, fibroblasts, when crowded, often locally align in parallel clusters.
Unlike the epithelial cells lining the body structures, fibroblasts do not form flat monolayers and are not restricted by a polarizing attachment to a basal lamina on one side, although they may contribute to basal lamina components in some situations (e.g. subepithelial myofibroblasts in intestine may secrete the α-2 chain carrying component of the laminin, which is absent only in regions of follicle-associated epithelia which lack the myofibroblast lining). Fibroblasts can also migrate slowly over substratum as individual cells, again in contrast to epithelial cells. While epithelial cells form the lining of body structures, it is fibroblasts and related connective tissues which sculpt the "bulk" of an organism.
The life span of a fibroblast, as measured in chick embryos, is 57 ± 3 days.[3]
Relationship with fibrocytes
Fibroblasts and fibrocytes are two states of the same cells, the former being the activated state, the latter the less active state, concerned with maintenance and tissue metabolism. Currently, there is a tendency to call both forms fibroblasts. The suffix "-blast" is used in cellular biology to denote a stem cell or a cell in an activated state of metabolism.
Fibroblasts are morphologically heterogeneous with diverse appearances depending on their location and activity. Though morphologically inconspicuous, ectopically transplanted fibroblasts can often retain positional memory of the location and tissue context where they had previously resided, at least over a few generations.[4] This remarkable behavior may lead to discomfort in the rare event that they stagnate there excessively.
Development
The main function of fibroblasts is to maintain the structural integrity of connective tissues by continuously secreting precursors of the extracellular matrix. Fibroblasts secrete the precursors of all the components of the extracellular matrix, primarily the ground substance and a variety of fibers. The composition of the extracellular matrix determines the physical properties of connective tissues.
Like other cells of connective tissue, fibroblasts are derived from primitive mesenchyme. Thus they express the intermediate filament protein vimentin, a feature used as a marker to distinguish their mesodermal origin.[5] However, this test is not specific as epithelial cells cultured in vitro on adherent substratum may also express vimentin after some time.
In certain situations epithelial cells can give rise to fibroblasts, a process called epithelial-mesenchymal transition (EMT).
Conversely, fibroblasts in some situations may give rise to epithelia by undergoing a mesenchymal to epithelial transition (MET) and organizing into a condensed, polarized, laterally connected true epithelial sheet. This process is seen in many developmental situations (e.g. nephron and notocord development), as well as in wound healing and tumorigenesis.
Function
Fibroblasts make collagen fibres, glycosaminoglycans, reticular and elastic fibers, Growing individuals' fibroblasts are dividing and synthesizing ground substance. Tissue damage stimulates fibrocytes and induces the production of fibroblasts.
Inflammation
Besides their commonly known role as structural components, fibroblasts play a critical role in an immune response to a tissue injury. They are early players in initiating inflammation in the presence of invading microorganisms. They induce chemokine synthesis through the presentation of receptors on their surface. Immune cells then respond and initiate a cascade of events to clear the invasive microorganisms. Receptors on the surface of fibroblasts also allow regulation of hematopoietic cells and provide a pathway for immune cells to regulate fibroblasts.[6]
Tumour mediation
Fibroblasts, like the tumor-associated host fibroblasts (TAF), play a crucial role in immune regulation through TAF-derived extracellular matrix (ECM) components and modulators. TAF are known to be significant in the inflammatory response as well as immune suppression in tumors. TAF-derived ECM components cause alterations in the ECM composition and initiate the ECM remodeling.[7] The ECM remodeling is described as changes in the ECM as a result of enzyme activity which can lead to degradation of the ECM. Immune regulation of tumors is largely determined by the ECM remodeling because the ECM is responsible for regulating a variety of functions, such as proliferation, differentiation, and morphogenesis of vital organs.[8] In many tumor types, especially those related to the epithelial cells, ECM remodeling is common. Examples of TAF-derived ECM components include Tenascin and Thrombospondin-1 (TSP-1), which can be found in sites of chronic inflammation and carcinomas respectively.[7]
Immune regulation of tumors can also occur through the TAF-derived modulators. Although these modulators may sound similar to the TAF-derived ECM components, they differ in the sense that they are responsible for the variation and turnover of the ECM. Cleaved ECM molecules can play a critical role in immune regulation. Proteases like matrix metalloproteineases (MMPs) and the uPA system are known to cleave the ECM. These proteases are derived from fibroblasts.[7]
Secondary actions
Mouse embryonic fibroblasts (MEFs) are often used as "feeder cells" in human embryonic stem cell research. However, many researchers are gradually phasing out MEFs in favor of culture media with precisely defined ingredients of exclusively human derivation. Further, the difficulty of exclusively using human derivation for media supplements is most often solved by use of "defined media" where the supplements are synthetic and achieve the primary goal of eliminating the chance of contamination from derivative sources.
In view of the clinical application of stem cell derived tissues, the use of human fibroblast as feeders has been studied[9]. Whereas the fibroblasts are usually used to maintain pluripotency of the stem cells, they can also be used to facilitate development of the stem cells into specific type of cells such as cardiomyocytes[10].
See also
References
- "Fibroblast". Genetics Home Reference. U.S. National Library of Medicine. 2014-05-05. Retrieved 2014-05-10.
- "Fibroblasts". Retrieved 16 August 2018.
- Weissman-Shomer P, Fry M (1975). "Chick embryo fibroblasts senscence in vitro: pattern of cell division and life span as a function of cell density". Mechanisms of Ageing and Development. 4 (2): 159–66. doi:10.1016/0047-6374(75)90017-2. PMID 1152547.
- Advances in Extracellular Space Research and Application: 2013 Edition. Scholarly Editions. 21 June 2013. p. 251. ISBN 9781481682626.
- Dave JM, Bayless KJ (May 2014). "Vimentin as an integral regulator of cell adhesion and endothelial sprouting". Microcirculation. 21 (4): 333–44. doi:10.1111/micc.12111. PMID 24387004.
- Smith RS, Smith TJ, Blieden TM, Phipps RP (August 1997). "Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation". The American Journal of Pathology. 151 (2): 317–22. PMC 1858004. PMID 9250144.
- Silzle T, Randolph GJ, Kreutz M, Kunz-Schughart LA (January 2004). "The fibroblast: sentinel cell and local immune modulator in tumor tissue". International Journal of Cancer. 108 (2): 173–80. doi:10.1002/ijc.11542. PMID 14639599.
- Bonnans C, Chou J, Werb Z (December 2014). "Remodelling the extracellular matrix in development and disease". Nature Reviews. Molecular Cell Biology. 15 (12): 786–801. doi:10.1038/nrm3904. PMC 4316204. PMID 25415508.
- Desai N, Rambhia P, Gishto A (February 2015). "Human embryonic stem cell cultivation: historical perspective and evolution of xeno-free culture systems". Reproductive Biology and Endocrinology. 13 (1): 9. doi:10.1186/s12958-015-0005-4. PMC 4351689. PMID 25890180.
- Matsuda Y, Takahashi K, Kamioka H, Naruse K (September 2018). "Human gingival fibroblast feeder cells promote maturation of induced pluripotent stem cells into cardiomyocytes". Biochemical and Biophysical Research Communications. 503 (3): 1798–1804. doi:10.1016/j.bbrc.2018.07.116. PMID 30060947.
External links
| Wikimedia Commons has media related to Fibroblasts. |
- UIUC Histology Subject 240
- MedEd at Loyola Histo/practical/ctproper/hp3-15.html