Famciclovir
Famciclovir is a guanosine analogue antiviral drug used for the treatment of various herpesvirus infections, most commonly for herpes zoster (shingles). It is a prodrug form of penciclovir with improved oral bioavailability. Famciclovir is marketed under the trade name Famvir (Novartis).
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌfæmˈsaɪkloʊˌvɪər/[1] |
| Trade names | Famvir |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a694038 |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 75–77% |
| Protein binding | 20–25% |
| Metabolism | Hepatic, circulation, intestinal wall (to penciclovir) |
| Elimination half-life | 2–2.3 hours |
| Excretion | Renal, faecal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.713 |
| Chemical and physical data | |
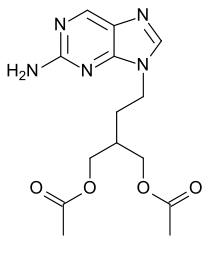
| Formula | C14H19N5O4 |
| Molar mass | 321.332 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 103 °C (217 °F) |
SMILES
| |
InChI
| |
| (verify) | |
Famciclovir was patented in 1983 and approved for medical use in 1994.[2][3] In 2007, the United States Food and Drug Administration approved the first generic version of famciclovir. Generic tablets are manufactured by TEVA Pharmaceuticals and Mylan Pharmaceuticals.[4][5]
Medical uses
Famciclovir is indicated for the treatment of herpes zoster (shingles),[6] treatment of herpes simplex virus 2 (genital herpes),[7] herpes labialis (cold sores) in immunocompetent patients[8] and for the suppression of recurring episodes of herpes simplex virus 2. It is also indicated for treatment of recurrent episodes of herpes simplex in HIV patients.
Adverse effects
Side effects: mild to extreme stomach upset, headaches, mild fever.
Herpes
Early treatment
Several studies in humans and mice provide evidence that early treatment with famciclovir soon after the first infection with herpes can significantly lower the chance of future outbreaks. Use of famciclovir in this manner has been shown to reduce the amount of latent virus in the neural ganglia compared to no treatment or treatment with valaciclovir.[9][10][11] A review of human subjects treated for five days with famciclovir 250 mg three times daily during their first herpes episode found that only 4.2 percent experienced a recurrence within six months after the first outbreak, a fivefold decrease compared to the 19 percent recurrence in acyclovir-treated patients.[12] Neither drug affected latency if treatment was delayed for several months.[13]
See also
References
- "Famciclovir". Merriam-Webster Dictionary. Retrieved 2016-01-22.
- Long, Sarah S.; Pickering, Larry K.; Prober, Charles G. (2012). Principles and Practice of Pediatric Infectious Disease. Elsevier Health Sciences. p. 1502. ISBN 1437727026.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 504. ISBN 9783527607495.
- "Recent Product Launches, Teva Pharmaceuticals USA". Archived from the original on 2011-11-08. Retrieved 2008-02-21.
- "Mylan Launches Generic Version of Famvir® Tablets" (Press release). Mylan. 20 April 2011. Archived from the original on July 23, 2011. Retrieved 21 April 2011.
- Tyring SK, Barbarash RA (1995). "Famciclovir for the Treatment of Acute Herpes Zoster: Effects on Acute Disease and Postherpetic Neuralgia". Annals of Internal Medicine. 123 (2): 89–96. doi:10.7326/0003-4819-123-2-199507150-00002. PMID 7778840.
- Luber AD, Flaherty JF (1996). "Famciclovir for Treatment of Herpesvirus Infections". Annals of Pharmacotherapy. 30 (9): 978–985. doi:10.1177/106002809603000913. PMID 8876860.
- Spruance SL, Bodsworth N (2006). "Single-Dose, Patient-Initiated Famciclovir: A Randomized, Double-Blind, Placebo-Controlled Trial for Episodic Treatment of Herpes Labialis". Journal of the American Academy of Dermatology. 55 (1): 47–53. doi:10.1016/j.jaad.2006.02.031. PMID 16781291.
- The effects of antiviral therapy on the distribution of herpes simplex virus type 1 to ganglionic neurons and its consequences during, immediately following and several months after treatment" Archived 2010-10-04 at the Wayback Machine"
- Famciclovir and Valaciclovir Differ in the Prevention of Herpes Simplex Virus Type 1 Latency in Mice: a Quantitative Study""
- Persistence of Infectious Herpes Simplex Virus Type 2 in the Nervous System in Mice after Antiviral Chemotherapy""
- Observation May Indicate A Possible Clinical Effect On Latency""
- Thackray AM, Field HJ. (1996). "Differential effects of famciclovir and valaciclovir on the pathogenesis of herpes simplex virus in a murine infection model including reactivation from latency". J. Infect. Dis. 173 (2): 291–299. doi:10.1093/infdis/173.2.291. PMID 8568288.