Ethyl eicosapentaenoic acid
Ethyl eicosapentaenoic acid (E-EPA, icosapent ethyl) is a medication used to treat hypertriglyceridemia. It is used in combination with changes in diet in adults with hypertriglyceridemia ≥ 150 mg/dL.[1]
 | |
| Names | |
|---|---|
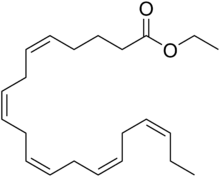
| IUPAC name
Ethyl (5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoate | |
| Other names
Eicosapentaenoic acid ethyl ester; Ethyl eicosapentaenoate; Eicosapent; Icosapent ethyl; EPA ethyl ester; E-EPA | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C22H34O2 |
| Molar mass | 330.512 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is made from the omega-3 fatty acid eicosapentaenoic acid (EPA). It was the second class of fish oil-based medications to be approved in the United States 2012.[1] These fish oil drugs are similar to fish oil dietary supplements but the ingredients are better controlled.
Medical use
E-EPA is used in addition to changes in diet to reduce triglyceride levels in adults with severe (≥ 500 mg/dL) hypertriglyceridemia.[2] Or it may be used in hypertriglyceridemia ≥ 150 mg/dL in those with risk factors for heart disease.[1]
Intake of large doses (2.0 to 4.0 g/day) of long-chain omega-3 fatty acids as prescription drugs or dietary supplements are generally required to achieve significant (> 15%) lowering of triglycerides, and at those doses the effects can be significant (from 20% to 35% and even up to 45% in individuals with levels greater that 500 mg/dL). It appears that both eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) lower triglycerides, however, DHA alone appears to raise low-density lipoprotein (the variant which drives atherosclerosis; sometimes very inaccurately called: "bad cholesterol") and LDL-C values (always only a calculated estimate; not measured by labs from person's blood sample for technical and cost reasons), whilst EPA alone does not and instead lowers the parameters aforementioned.[3]
Other fish-oil based drugs
There are other omega-3 fish oil based drugs on the market that have similar uses and mechanisms of action.[4]
- Omega-3 acid ethyl esters (brand names Omarcor or Lovaza,[5] Omtryg,[6] and as of March 2016, four generic versions.[7]
- Omega-3 carboxylic acids (Epanova)[8]
Dietary supplements
There are many fish oil dietary supplements on the market.[9] There appears to be little difference in effect between dietary supplements and prescription forms of omega-3 fatty acids, but EPA and DHA ethyl esters (prescription forms) work less well when taken on an empty stomach or with a low-fat meal.[3] The ingredients of dietary supplements are not as carefully controlled as prescription products and have not been fixed and tested in clinical trials, as prescription drugs have,[10] and the prescription forms are more concentrated, requiring fewer capsules to be taken and increasing the likelihood of compliance.[9]
Side effects
Special caution should be taken with people who have with fish and shellfish allergies.[2] In addition, as with other omega-3 fatty acids, taking E-EPA puts people who are on anticoagulants at risk for prolonged bleeding time.[2][3] The most commonly reported side effect in clinical trials has been joint pain; some people also reported pain in their mouth or throat.[2] E-EPA has not been tested in pregnant women is rated pregnancy category C; it is excreted in breast milk and the effects on infants are not known.[2]
Pharmacology
After ingestion, E-EPA is metabolized to EPA. EPA is absorbed in the small intestine and enters circulation. Peak plasma concentration occurs about 5 hours after ingestion and the half-life is about 89 hours. EPA is metabolized mostly in the liver like other dietary fatty acids.[2]
Mechanism of action
EPA, the active metabolite of E-EPA, like other omega-3 fatty acid based drugs, appears to reduce production of triglycerides in the liver, and to enhance clearance of triglycerides from circulating very low-density lipoprotein (VLDL) particles; the way it does that is not clear, but potential mechanisms include increased breakdown of fatty acids; inhibition of diglyceride acyltransferase which is involved in biosynthesis of triglycerides in the liver; and increased activity of lipoprotein lipase in blood.[2][4]
Chemistry
E-EPA is an ethyl ester of eicosapentaenoic acid, which is an omega-3 fatty acid.[2]
History
In July 2012, the US Food and Drug Administration approved E-EPA for severe hypertriglyceridemia as an adjunct to dietary measures; Amarin Corporation had developed the drug.[11] Amarin Corporation, challenged the FDA's authority to limit its ability to market the drug for off-label use and won its case on appeal in 2012, changing the way the FDA regulates the marketing of medication.
E-EPA was the second fish-oil drug to be approved, after omega-3 acid ethyl esters (GlaxoSmithKline's Lovaza which was approved in 2004[12]) and sales were not as robust as Amarin had hoped. The labels for the two drugs were similar, but doctors prescribed Lovaza for people who had triglycerides lower than 500 mg/dL based on some clinical evidence. Amarin wanted to actively market E-EPA for that population as well which would have greatly expanded its revenue, and applied to the FDA for permission to do so in 2013, which the FDA denied.[13] In response, in May 2015 Amarin sued the FDA for infringing its First Amendment rights,[14] and in August 2015 a judge ruled that the FDA could not "prohibit the truthful promotion of a drug for unapproved uses because doing so would violate the protection of free speech."[15] The ruling left open the question of what the FDA would allow Amarin to say about E-EPA, and in March 2016 the FDA and Amarin agreed that Amarin would submit specific marketing material to the FDA for the FDA to review, and if the parties disagreed on whether the material was truthful, they would seek a judge to mediate.[16]
References
- Commissioner, Office of the (17 December 2019). "FDA approves use of drug to reduce risk of cardiovascular events in certain adult patient groups". FDA. Retrieved 18 December 2019.
- Icosapent ethyl Label Last revised June 2015. Check for updates at FDA label index page here
- Jacobson TA, et al, NLA Expert Panel. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2. J Clin Lipidol. 2015 Nov-Dec;9(6 Suppl):S1-S122.e1. PMID 26699442 Free full text
- Weintraub, HS (2014). "Overview of prescription omega-3 fatty acid products for hypertriglyceridemia". Postgrad Med. 126: 7–18. doi:10.3810/pgm.2014.11.2828. PMID 25387209. Archived from the original on 20 April 2015. Retrieved 20 April 2015.
- "University of Utah Pharmacy Services (15 August 2007) "Omega-3-acid Ethyl Esters Brand Name Changed from Omacor to Lovaza"". Archived from the original on 3 March 2016. Retrieved 1 April 2016.
- Omtryg Label Revised April 2014
- FDA Omega-3 acid ethyl esters products Page accessed 31 March 2016
- "Epanova (omega-3-carboxylic acids)". CenterWatch. Retrieved 15 December 2014.
- Ito MK. A Comparative Overview of Prescription Omega-3 Fatty Acid Products. P T. 2015 Dec;40(12):826-57. PMID 26681905 Free PMC Article PMC 4671468
- Sweeney MET. Hypertriglyceridemia Pharmacologic Therapy for Medscape Drugs & Diseases, Ed. Khardori R. Updated: 14 April 2015, page accessed 1 April 2016
- CenterWatch Vascepa (icosapent ethyl) Page accessed 31 March 2016
- VHA Pharmacy Benefits Management Strategic Healthcare Group and the Medical Advisory Panel. October 2005 National PBM Drug Monograph Omega-3-acid ethyl esters (Lovaza, formerly Omacor)
- Matthew Herper for Forbes. 17 October 2013 Why The FDA Is Right To Block Amarin's Push To Market Fish Oil To Millions
- Thomas, Katie (7 May 2015). "Drugmaker Sues F.D.A. Over Right to Discuss Off-Label Uses". New York Times. Retrieved 17 May 2017.
- Andrew Pollack for the New York Times. 7 August 2015 Court Forbids F.D.A. From Blocking Truthful Promotion of Drug
- Katie Thomas for the New York Times. 8 March 2016 F.D.A. Deal Allows Amarin to Promote Drug for Off-Label Use