Estrone
Estrone (E1), also spelled oestrone, is a steroid, a weak estrogen, and a minor female sex hormone.[1] It is one of three major endogenous estrogens, the others being estradiol and estriol.[1] Estrone, as well as the other estrogens, are synthesized from cholesterol and secreted mainly from the gonads, though they can also be formed from adrenal androgens in adipose tissue.[2] Relative to estradiol, both estrone and estriol have far weaker activity as estrogens.[1] Estrone can be converted into estradiol, and serves mainly as a precursor or metabolic intermediate of estradiol.[1][3]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(8R,9S,13S,14S)-3-hydroxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-one | |
| Other names
Oestrone; E1; 3-Hydroxyestra-1,3,5(10)-trien-17-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.150 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C18H22O2 |
| Molar mass | 270.366 g/mol |
| Melting point | 254.5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In addition to its role as a natural hormone, estrone has been used as a medication, for instance in menopausal hormone therapy; for information on estrone as a medication, see the estrone (medication) article.
Biological activity
Estrone is an estrogen, specifically an agonist of the estrogen receptors ERα and ERβ.[1][4] It is a far less potent estrogen than is estradiol, and as such, is a relatively weak estrogen.[1][4][5] Given by subcutaneous injection in mice, estradiol is about 10-fold more potent than estrone and about 100-fold more potent than estriol.[6] According to one study, the relative binding affinities of estrone for the human ERα and ERβ were 4.0% and 3.5% of those estradiol, respectively, and the relative transactivational capacities of estrone at the ERα and ERβ were 2.6% and 4.3% of those of estradiol, respectively.[4] In accordance, the estrogenic activity of estrone has been reported to be approximately 4% of that of estradiol.[1] In addition to its low estrogenic potency, estrone, unlike estradiol and estriol, is not accumulated in estrogen target tissues.[1] Because estrone can be transformed into estradiol, most of the estrogenic potency of estrone in vivo is actually due to conversion into estradiol.[1] As such, estrone is considered to be a precursor or prohormone of estradiol.[3]
Clinical research has confirmed the nature of estrone as a relatively inert precursor of estradiol.[1][7][8][9] With oral administration of estradiol, the ratio of estradiol levels to estrone levels is about 5 times higher on average than under normal physiological circumstances in premenopausal women and with parenteral (non-oral) routes of estradiol.[1] Oral administration of menopausal replacement dosages of estradiol results in low, follicular phase levels of estradiol, whereas estrone levels resemble the high levels seen during the first trimester of pregnancy.[1][10][11] In spite of markedly elevated levels of estrone with oral estradiol but not with transdermal estradiol, clinical studies have shown that dosages of oral and transdermal estradiol achieving similar levels of estradiol possess equivalent and non-significantly different potency in terms of measures including suppression of luteinizing hormone and follicle-stimulating hormone levels, inhibition of bone resorption, and relief of menopausal symptoms such as hot flashes.[1][7][8][9] In addition, estradiol levels were found to correlate with these effects, while estrone levels did not.[7][8] These findings confirm that estrone has very low estrogenic activity, and also indicate that estrone does not diminish the estrogenic activity of estradiol.[1][7][8][9] This contradicts some in vitro research suggesting that estrone might be able to partially antagonize the actions of estradiol.[12][13][14]
Biochemistry
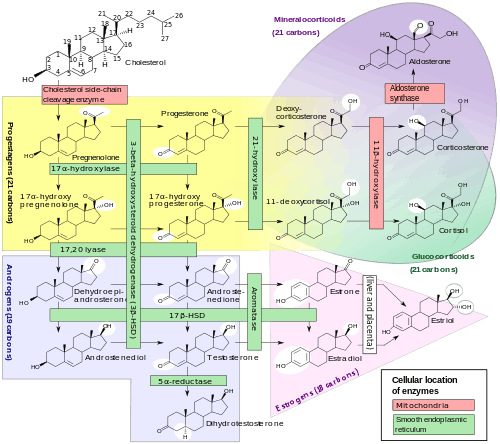
Biosynthesis
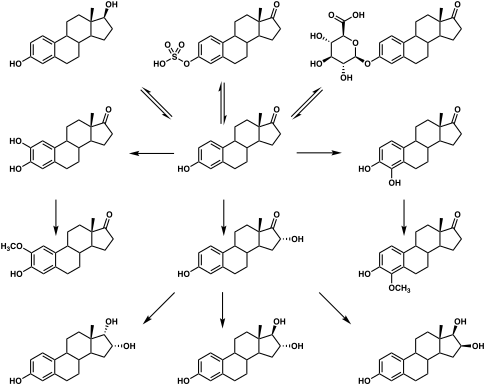
Estrone is biosynthesized from cholesterol. The principal pathway involves androstenedione as an intermediate, with androstenedione being transformed into estrone by the enzyme aromatase. This reaction occurs in both the gonads and in certain other tissues, particularly adipose tissue, and estrone is subsequently secreted from these tissues.[2] In addition to aromatization of androstenedione, estrone is also formed reversibly from estradiol by the enzyme 17β-hydroxysteroid dehydrogenase (17β-HSD) in various tissues, including the liver, uterus, and mammary gland.[1]
Distribution
Estrone is bound approximately 16% to sex hormone-binding globulin (SHBG) and 80% to albumin in the circulation,[1] with the remainder (2.0 to 4.0%) circulating freely or unbound.[16] It has about 24% of the relative binding affinity of estradiol for SHBG.[1] As such, estrone is relatively poorly bound to SHBG.[17]
Metabolism
Estrone is conjugated into estrogen conjugates such as estrone sulfate and estrone glucuronide by sulfotransferases and glucuronidases, and can also be hydroxylated by cytochrome P450 enzymes into catechol estrogens such as 2-hydroxyestrone and 4-hydroxyestrone or into estriol.[1] Both of these transformations take place predominantly in the liver.[1] Estrone can also be reversibly converted into estradiol by 17β-HSD.[1]
Excretion
Estrone is excreted in urine in the form of estrogen conjugates such as estrone sulfate.[1]
Chemistry
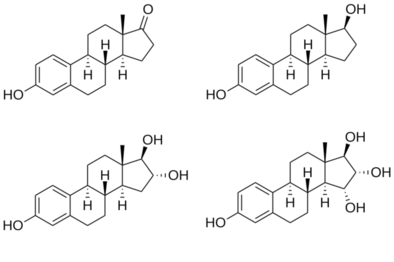
Structures of major endogenous estrogens
|
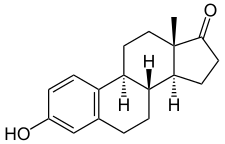
Estrone, also known as estra-1,3,5(10)-trien-3-ol-17-one, is a naturally occurring estrane steroid with double bonds at the C1, C3, and C5 positions, a hydroxyl group at the C3 position, and a ketone group at the C17 position. The name estrone was derived from the chemical terms estrin (estra-1,3,5(10)-triene) and ketone.
The chemical formula of estrone is C18H22O2 and its molecular weight is 270.366 g/mol. It is a white, odorless, solid crystalline powder, with a melting point of 254.5 °C (490 °F) and a specific gravity of 1.23.[21][22] Estrone is combustible at high temperatures, with the products carbon monoxide (CO) and carbon dioxide (CO2).[21]
Medical use
Estrone has been available as an injected estrogen for medical use, for instance in hormone therapy for menopausal symptoms, but it is now mostly no longer marketed.[23]
History
Estrone was the first steroid hormone to be discovered.[24][25] It was discovered in 1929 independently by the American scientists Edward Doisy and Edgar Allen and the German biochemist Adolf Butenandt, although Doisy and Allen isolated it two months before Butenandt.[24][26][27] They isolated and purified estrone in crystalline form from the urine of pregnant women.[26][27][28] Doisy and Allen named it theelin, while Butenandt named it progynon and subsequently referred to it as folliculin in his second publication on the substance.[27][29] Butenandt was later awarded the Nobel Prize in 1939 for the isolation of estrone and his work on sex hormones in general.[28][30] The molecular formula of estrone was known by 1931,[31] and its chemical structure had been determined by Butenandt by 1932.[27][26] Following the elucidation of its structure, estrone was additionally referred to as ketohydroxyestrin or oxohydroxyestrin,[32][33] and the name estrone, on the basis of its C17 ketone group, was formally established in 1932 at the first meeting of the International Conference on the Standardization of Sex Hormones in London.[34][35]
References
- Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Hornstein T, Schwerin JL (1 January 2012). Biology of Women. Cengage Learning. pp. 369–. ISBN 978-1-285-40102-7.
- van Keep PA, Utian WH, Vermeulen A (6 December 2012). The Controversial Climacteric: The workshop moderators' reports presented at the Third International Congress on the Menopause, held in Ostend, Belgium, in June 1981, under the auspices of the International Menopause Society. Springer Science & Business Media. p. 92. ISBN 978-94-011-7253-0.
- Escande A, Pillon A, Servant N, Cravedi JP, Larrea F, Muhn P, Nicolas JC, Cavaillès V, Balaguer P (May 2006). "Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta". Biochemical Pharmacology. 71 (10): 1459–69. doi:10.1016/j.bcp.2006.02.002. PMID 16554039.
- Ruggiero RJ, Likis FE (2002). "Estrogen: physiology, pharmacology, and formulations for replacement therapy". Journal of Midwifery & Women's Health. 47 (3): 130–8. doi:10.1016/s1526-9523(02)00233-7. PMID 12071379.
- A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 548–. ISBN 978-3-642-96158-8.
- Selby P, McGarrigle HH, Peacock M (March 1989). "Comparison of the effects of oral and transdermal oestradiol administration on oestrogen metabolism, protein synthesis, gonadotrophin release, bone turnover and climacteric symptoms in postmenopausal women". Clinical Endocrinology. 30 (3): 241–9. doi:10.1111/j.1365-2265.1989.tb02232.x. PMID 2512035.
- Powers MS, Schenkel L, Darley PE, Good WR, Balestra JC, Place VA (August 1985). "Pharmacokinetics and pharmacodynamics of transdermal dosage forms of 17 beta-estradiol: comparison with conventional oral estrogens used for hormone replacement". American Journal of Obstetrics and Gynecology. 152 (8): 1099–106. doi:10.1016/0002-9378(85)90569-1. PMID 2992279.
- Fåhraeus L, Larsson-Cohn U (December 1982). "Oestrogens, gonadotrophins and SHBG during oral and cutaneous administration of oestradiol-17 beta to menopausal women". Acta Endocrinologica. 101 (4): 592–6. doi:10.1530/acta.0.1010592. PMID 6818806.
- Wright JV (December 2005). "Bio-identical steroid hormone replacement: selected observations from 23 years of clinical and laboratory practice". Annals of the New York Academy of Sciences. 1057: 506–24. doi:10.1196/annals.1356.039. PMID 16399916.
- Friel PN, Hinchcliffe C, Wright JV (March 2005). "Hormone replacement with estradiol: conventional oral doses result in excessive exposure to estrone". Alternative Medicine Review. 10 (1): 36–41. PMID 15771561.
- Kloosterboer, HJ; Schoonen, WG; Verheul, HA (11 April 2008). "Proliferation of Breast Cells by Steroid Hormones and Their Metabolites". In Pasqualini, Jorge R (ed.). Breast Cancer: Prognosis, Treatment, and Prevention. CRC Press. pp. 343–366. ISBN 978-1-4200-5873-4.
- Sasson S, Notides AC (July 1983). "Estriol and estrone interaction with the estrogen receptor. II. Estriol and estrone-induced inhibition of the cooperative binding of [3H]estradiol to the estrogen receptor". The Journal of Biological Chemistry. 258 (13): 8118–22. PMID 6863280.
- Lundström E, Conner P, Naessén S, Löfgren L, Carlström K, Söderqvist G (2015). "Estrone - a partial estradiol antagonist in the normal breast". Gynecological Endocrinology. 31 (9): 747–9. doi:10.3109/09513590.2015.1062866. PMID 26190536.
- Häggström M, Richfield D (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
- Jameson JL, De Groot LJ (18 May 2010). Endocrinology – E-Book: Adult and Pediatric. Elsevier Health Sciences. pp. 2813–. ISBN 978-1-4557-1126-0.
- H.J. Buchsbaum (6 December 2012). The Menopause. Springer Science & Business Media. pp. 62, 64. ISBN 978-1-4612-5525-3.
- Buchsbaum HJ, ed. (2012). The Menopause (Clinical Perspectives in Obstetrics and Gynecology). New York, NY: Springer Science & Business Media. p. 64. ISBN 9781461255253.
- Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric : the Journal of the International Menopause Society. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- "EC 2.4.1.17 – glucuronosyltransferase and Organism(s) Homo sapiens". BRENDA. Technische Universität Braunschweig. January 2018. Retrieved 10 August 2018.
- "Material Safety Data Sheet Estrone" (PDF). ScienceLab.com. Retrieved 21 February 2013.
- "Estrone -PubChem". National Center for Biotechnology Information. Retrieved 6 September 2009.
- "Drugs@FDA: FDA Approved Drug Products".
- Vern L. Bullough (19 May 1995). Science In The Bedroom: A History Of Sex Research. Basic Books. pp. 128–. ISBN 978-0-465-07259-0.
When Allen and Doisy heard about the [Ascheim-Zondek test for the diagnosis of pregnancy], they realized there was a rich and easily handled source of hormones in urine from which they could develop a potent extract. [...] Allen and Doisy's research was sponsored by the committee, while that of their main rival, Adolt Butenandt (b. 1903) of the University of Gottingen was sponsored by a German pharmaceutical firm. In 1929, both terms announced the isolation of a pure crystal female sex hormone, estrone, in 1929, although Doisy and Allen did so two months earlier than Butenandt.27 By 1931, estrone was being commercially produced by Parke Davis in this country, and Schering-Kahlbaum in Germany. Interestingly, when Butenandt (who shared the Nobel Prize for chemistry in 1939) isolated estrone and analyzed its structure, he found that it was a steroid, the first hormone to be classed in this molecular family.
- Nielsch U, Fuhrmann U, Jaroch S (30 March 2016). New Approaches to Drug Discovery. Springer. pp. 7–. ISBN 978-3-319-28914-4.
The first steroid hormone was isolated from the urine of pregnant women by Adolf Butenandt in 1929 (estrone; see Fig. 1) (Butenandt 1931).
- Fritz F. Parl (2000). Estrogens, Estrogen Receptor and Breast Cancer. IOS Press. pp. 4–5. ISBN 978-0-9673355-4-4.
[Doisy] focused his research on the isolation of female sex hormones from hundreds of gallons of human pregnancy urine based on the discovery by Ascheim and Zondeck in 1927 that the urine of pregnant women possessed estrogenic activity [9]. In the summer of 1929, Doisy succeeded in the isolated of estrone (named by him theelin), simultaneously with but independent of Adolf Butenandt of the University of Gottingen in Germany. Doisy presented his results on the crystallization of estrone at the XIII International Physiological Congress in Boston in August 1929 [10].
- James K. Laylin (30 October 1993). Nobel Laureates in Chemistry, 1901–1992. Chemical Heritage Foundation. pp. 255–. ISBN 978-0-8412-2690-6.
Adolt Friedrich Johann Butenandt was awarded the Nobel Prize in chemistry in 1939 "for his work on sex hormones"; [...] In 1929 Butenandt isolated estrone [...] in pure crystalline form. [...] Both Butenandt and Edward Doisy isolated estrone simultaneously but independently in 1929. [...] Butenandt took a big step forward in the history of biochemistry when he isolated estrone from the urine of pregnant women. [...] He named it "progynon" in his first publication, and then "folliculine", [...] By 1932, [...] he could determine its chemical structure, [...]
- Arthur Greenberg (14 May 2014). Chemistry: Decade by Decade. Infobase Publishing. pp. 127–. ISBN 978-1-4381-0978-7.
Rational chemical studies of human sex hormones began in 1929 with Adolph Butenandt's isolation of pure crystalline estrone, the follicular hormone, from the urine of pregnant women. [...] Butenandt and Ruzicka shared the 1939 Nobel Prize in chemistry.
- A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 511–. ISBN 978-3-642-96158-8.
E. A. Doisy and A. Butenandt reported almost at the same time on the isolation of an estrogen-active substance in crystalline form from the urine of pregnant women. N. K. Adam suggested that this substance be named estrone because of the C-17-ketone group present (1933).
- Thom Rooke (1 January 2012). The Quest for Cortisone. MSU Press. pp. 54–. ISBN 978-1-60917-326-5.
In 1929 the first estrogen, a steroid called "estrone," was isolated and purified by Doisy; he later won a Nobel Prize for this work.
- D. Lynn Loriaux (23 February 2016). A Biographical History of Endocrinology. Wiley. pp. 345–. ISBN 978-1-119-20247-9.
- Campbell, A. D. (1933). "Concerning Placental Hormones and Menstrual Disorders". Annals of Internal Medicine. 7 (3): 330. doi:10.7326/0003-4819-7-3-330. ISSN 0003-4819.
- Fluhmann CF (November 1938). "Estrogenic Hormones: Their Clinical Usage". California and Western Medicine. 49 (5): 362–6. PMC 1659459. PMID 18744783.
- Fritz MA, Speroff L (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 750–. ISBN 978-1-4511-4847-3.
In 1926, Sir Alan S. Parkes and C.W Bellerby coined the basic word "estrin" to designate the hormone or hormones that induce estrus in animals, the time when female mammals are fertile and receptive to males. [...] The terminology was extended to include the principal estrogens in humans, estrone, estradiol, and estriol, in 1932 at the first meeting of the International Conference on the Standardization of Sex Hormones in London, [...]
- Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 2–. ISBN 978-3-642-58616-3.
The structure of the estrogenic hormones was stated by Butenandt, Thayer, Marrian, and Hazlewood in 1930 and 1931 (see Butenandt 1980). Following the proposition of the Marrian group, the estrogenic hormones were given the trivial names of estradiol, estrone, and estriol. At the first meeting of the International Conference on the Standardization of Sex Hormones, in London (1932), a standard preparation of estrone was established. [...] The partial synthesis of estradiol and estrone from cholesterol and dehydroepiandrosterone was accomplished by Inhoffen and Howleg (Berlin 1940); the total synthesis was achieved by Anner and Miescher (Basel, 1948).